
A “predicate device” is a medical device that has been previously approved by the US Food and Drug Administration (US FDA) and is already on the market, which serves as a reference point for new medical devices seeking approval through the FDA’s 510(k) clearance pathway.
The subject device must be proven to be at least as safe and effective as the predicate device in terms of its intended use and technological characteristics. This comparison is known as “substantial equivalence” determination.
A new device does not need to be identical to the predicate device for it to be substantially equivalent to the predicate device.
How to Identify a Predicate Device?
FDA’s database provides a three-letter product code for each device classification. The FDA 510(k) database contains information on all devices cleared through the 510(k) process. Once you have the three-letter product code, you can get a list of every product, every company, and the trade name of every competitor or potential competitor that you want to look at. You can then do an in-depth analysis and comparison to narrow down a predicate device.
Below is a flowchart depicting the process of identifying and narrowing down a predicate device.
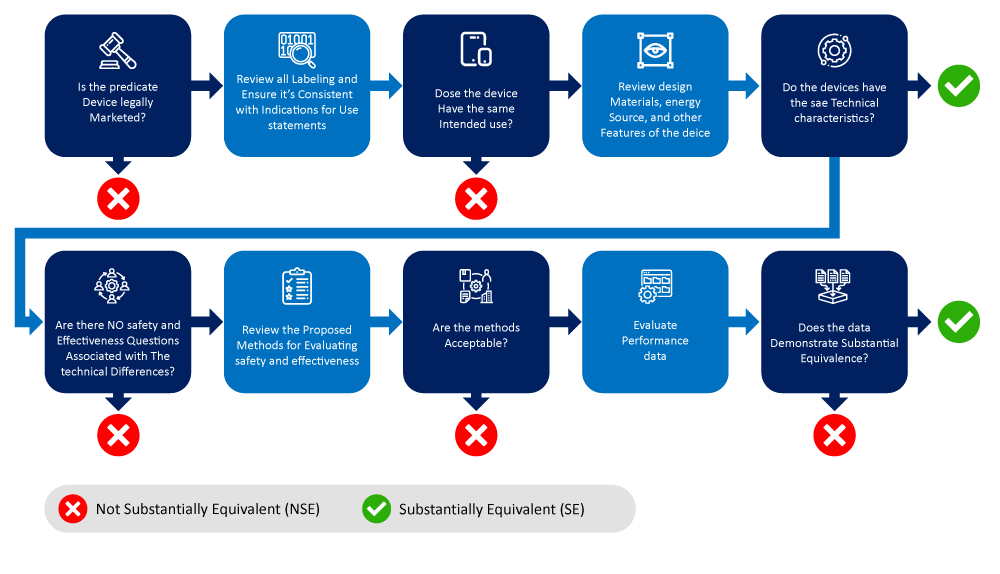
Factors to Consider When Determining the Predicate Device(s)
- Intended use: The intended use after the predicate device should be similar to that of the new device. For example, if the new device is intended to be used for heart monitoring, the predicate device should also be a heart monitoring device.
- Technological characteristics: The predicate device should be identical to the new device in terms of technological characteristics. Take, for instance, the design, materials used, and the method of operation that should be similar.
- Biocompatibility: Biocompatibility assessments of a medical device or component should not be limited to the raw materials used in the device and manufacturing processing, and additional chemicals should also be considered. This factor, however, doesn’t apply to IVDs.
- Latest technology: The predicate device should not be dated and should represent the latest medical technology.
The predicate device is a key factor in determining whether a new medical device can be brought to market through the 510(k) pathway. Choosing the wrong predicate device could result in a more expensive and time-consuming Regulatory approval process while choosing the right predicate device can help reduce the cost and time required to bring a new medical device to market. If the predicate device is not suitable, it could result in delays and additional expenses.
For assistance with the 510(k)-submission process of your medical device, schedule a call with Freyr Regulatory experts, who can help you navigate the procedures. Stay informed. Stay compliant.









