Non-clinical data plays a pivotal role in shaping the labeling of new pharmaceuticals by ensuring that drug product labels provide comprehensive information about the safety, efficacy, and appropriate use of the drug. This blog examines how non-clinical studies influence drug labeling, ensuring safety and efficacy for end-users.
Impact of non-clinical data on Drug Product Labeling
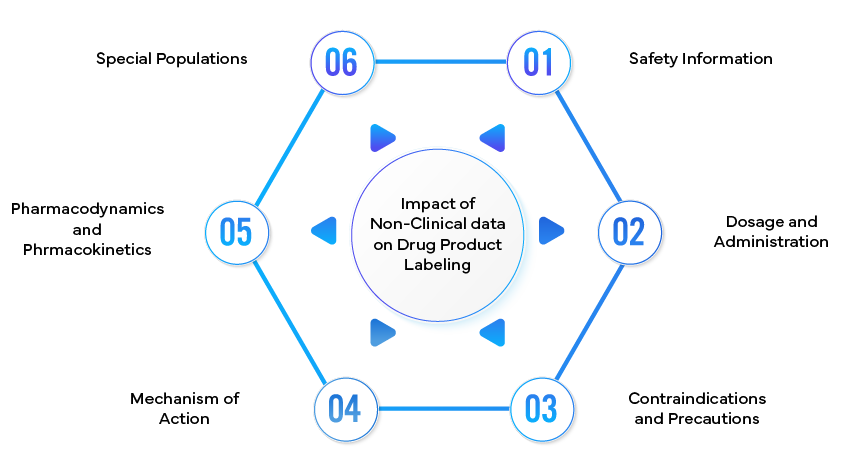
- Safety Information:
Influence: Non-clinical studies help in identifying potential adverse effects, safe dosage ranges, and interactions with other substances.
Outcome: This ensures that drug labels contain accurate safety warnings, adverse reactions, contraindications, and usage precautions. It helps healthcare providers make informed decisions and allows patients to understand potential risks.
- Dosage and Administration:
Influence: Non-clinical data helps determine the appropriate dosage regimens and administration routes. Studies on the absorption, distribution, metabolism, and excretion (ADME) of the drug contribute to understanding its behavior in the body.
Outcome: This facilitates the development of clear and precise dosing instructions on drug labels. Accurate dosage information is crucial for achieving therapeutic efficacy and minimizing adverse effects.
- Contraindications and Precautions:
Influence: Non-clinical studies identify contraindications and the necessary precautions based on findings related to drug interactions, effects in specific populations, and long-term safety.
Outcome: This enhances patient safety by providing comprehensive risk information, helping healthcare providers avoid prescribing the drug to unsuitable patients, and informing patients about conditions under which they should avoid using the drug.
- Mechanism of Action:
Influence: Non-clinical studies often elucidate the mechanism of action of a drug and detailed biochemical and physiological studies.
Outcome: Including the mechanism of action in the drug label helps healthcare providers understand how the drug works, which can influence prescribing decisions and patient management.
- Pharmacodynamics and Pharmacokinetics:
Influence: Non-clinical studies provide detailed pharmacodynamic and pharmacokinetic data, which describe the drug's effects on the body and how the body affects the drug over time.
Outcome: This information is crucial for labeling, as it informs about the onset, duration, and intensity of the drug’s effects, as well as its bioavailability, half-life, and elimination. This helps in optimizing dosing schedules and understanding the impact of various patient factors like age, gender, and renal or hepatic function.
- Special Populations:
Influence: Non-clinical studies may include investigations on specific populations, such as pregnant or lactating women, and pediatric or geriatric populations.
Outcome: This data ensures that the drug label includes essential information on the safety and efficacy of the drug in these special populations, guiding appropriate use and dosing adjustments.
Non-clinical data significantly impacts drug labeling, ensuring that labels provide accurate safety and usage information. Partnering with Regulatory experts ensures that non-clinical data is effectively translated into compliant and informative labels, supporting patient safety and drug efficacy. Partner with us to ensure your product labeling complies with its safety and efficacy for patients.