
Did you know An effective eDMS enhances compliance by centralizing and standardizing document management, reducing the risk of non-compliance by 40%. In the highly regulated life sciences industry, efficient document management is more than just a compliance requirement—it’s a strategic advantage. Regulatory affairs depend on timely, accurate submissions, and any misstep can lead to costly delays or non-compliance. Implementing an effective document management system like Freyr rDMS, not only enhances compliance but also reduces risk and drives innovation. Let’s dive into how its transforming regulatory processes, backed by compelling data.
Why an eDMS is Essential for Regulatory Compliance
In a sector where compliance is everything, staying ahead of regulatory requirements is crucial. By centralizing and standardizing document management, eDMS systems streamline workflows and provide regulatory teams with real-time access to accurate, validated information, mitigating the risk of errors and ensuring faster decision-making.
Key Benefits: Before and after adoption of a document management system
| Aspect | Without Freyr rDMS | With Freyr rDMS |
| Data Integrity | High risk of data inconsistencies due to manual entry | Automated validation ensures real-time updates and data accuracy |
| Regulatory Intelligence | Limited strategic insights from regulatory data | Proactive compliance with integrated regulatory intelligence |
| Global Compliance | Complexities in managing multiple regions' requirements | Centralized updates simplify multi-regional compliance |
| Collaboration | Siloed teams with limited sharing capabilities | Global teams collaborate seamlessly in real-time |
| Operational Agility | Slow response to regulatory shifts | Streamlined processes increase agility |
| Cost Efficiency | High costs due to inefficiencies | Reduced costs with automation and less manual work |
| Regulatory Submissions | Manual, error-prone submissions | Automated, accurate, and timely submissions |
| Innovation Support | Regulatory hurdles slow innovation | Rapid adaptation to new regulations fosters innovation |
With such tangible benefits, it's clear that Freyr rDMS is a game-changer for regulatory affairs. It enables organizations to transform compliance from a reactive task into a strategic one.
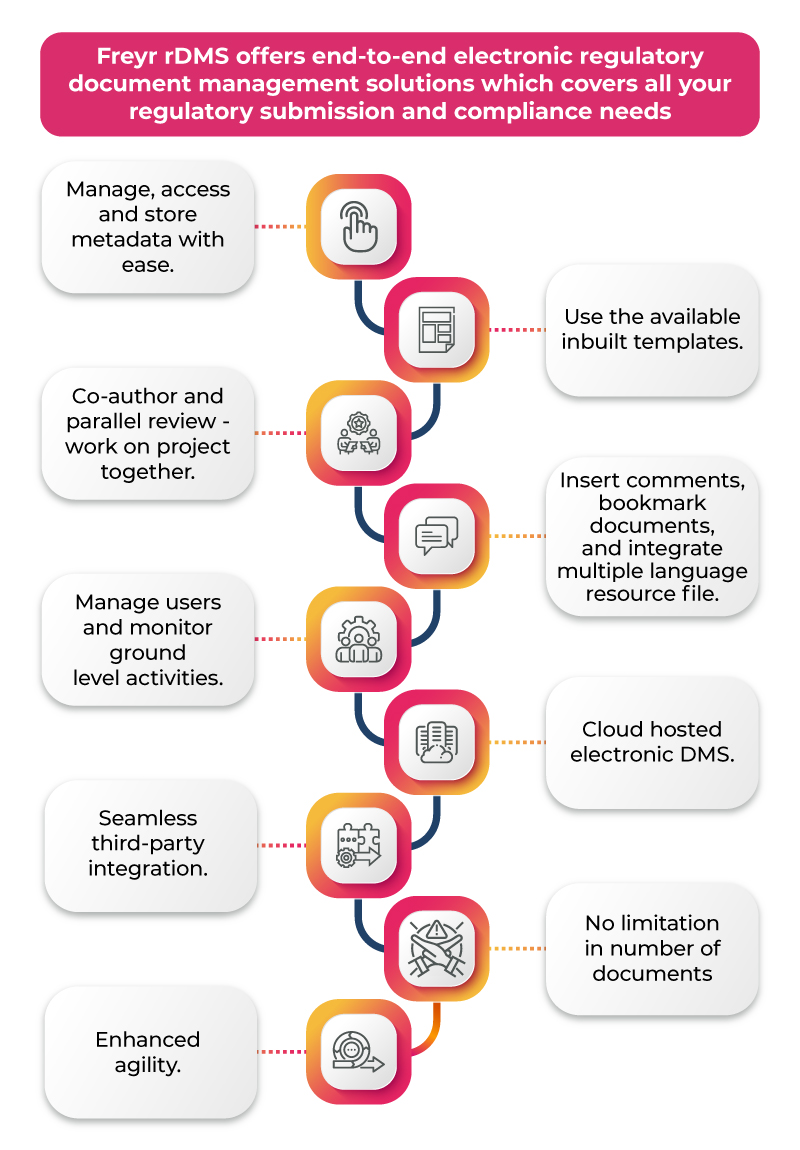
The Technological Impact of an eDMS on the Regulatory Journey
Modern DMS solutions leverage cutting-edge technology, ensuring both compliance and efficiency. Here’s a closer look at three key technological advancements that drive the impact of eDMS in regulatory affairs:
Cloud-Based Solutions
Cloud technology is revolutionizing the way regulatory documents are managed.
- Scalability: Easily adjust to growing regulatory demands without additional infrastructure costs.
- Remote Access: Teams can collaborate from anywhere in the world, ensuring faster submissions and global compliance.
Blockchain for Enhanced Security
Security is paramount in the life sciences industry. Blockchain adds a new layer of trust and transparency.
- Immutable Records: Once entered, documents cannot be altered, ensuring high data integrity.
- Transparent Audit Trails: The technology creates audit trails that make the document’s history fully transparent and secure, ensuring accountability.
Regulatory Intelligence Tools
Staying compliant is not just about following regulations, it’s about anticipating changes.
- Real-Time Updates: Integration of real-time regulatory intelligence ensures that organizations remain compliant with changing regulations across multiple regions.
- Strategic Insights: These tools allow companies to leverage data for better decision-making, transforming regulatory affairs into a source of competitive advantage.
A Global Perspective: Streamlining Regional Compliance
Regulatory requirements vary by region, and the ability to adapt is crucial. An effective rDMS supports submissions across North America, Europe, Asia, and more, ensuring smooth global product launches without delays. This global reach ensures that your products meet regulatory requirements worldwide, reducing the risk of market entry delays by 35%.
Final Thoughts: From Compliance to Strategic Innovation
DMS technology transforms regulatory functions from compliance-driven to innovation-led. Automated workflows for document creation, review, approval, and distribution can reduce the time spent on these tasks by 50%. Transform your regulatory affairs into a proactive, strategic force with Freyr rDMS. Streamline your document management and ensure compliance with end-to-end monitoring, inbuilt audit capabilities and is migration friendly. Explore Freyr rDMS today to enhance efficiency and drive growth. Contact us.









