
The Health Authorities of all countries, with Canada being an exception, require all foreign medical device manufacturers to appoint a local, in-country or Authorized representative. The duty of a legal representative is to represent the manufacturer and the device in the targeted country and to liaison between Health Authorities and the foreign manufacturers. There is country-specific jargon for a local representative. The local representative in the USA is called as a US Agent, and that in the UK is referred to as UKRP (United Kingdom Responsible Person). In Sri Lanka, the local representative is termed as Sri Lanka Local Agent or Sri Lanka Authorized representative, who also is the Marketing Authorization Holder (MAH) for the device.
The foreign medical device manufacturers may opt to appoint their distributor as a local agent, who by default would also be the Market Authorization Holder (MAH). However, it is recommended to appoint a third-party service provider with no business conflicts as your MAH. The pros of appointing the independent third-party service provider as your MAH are mentioned below. A MAH shall:
- Be competent in country Regulatory affairs and PMS requirements
- Achieve business goals by appointing multiple distributors for market penetration
- Ability to focus on Regulatory compliance
- Technically competent to address the queries raised by the Agency
- Common labels for a single MAH throughout the country
- Avoid the nuances of having to update labels in case of change in a distributor, who also would be acting as the MAH/local agent
In few countries, for e.g., in India, there is no provision to transfer the Market authorization and require a new application to be submitted. In contrast, in Sri Lanka, The National Medicine Regulatory Authority (NMRA) allows for MAH transfer. The major challenge that foreign device manufacturers face while changing the MAH is the requirement to submit a No Objection Certificate/Letter (NoC/NoL) from the current active MAH. NMRA also facilitates a MAH transfer, even when a MAH is reluctant to issue a NOC.
The change in the name and/or address of the Sri Lanka MAH is not considered as a MA transfer if the holder is the same person/entity, and such change should be notified through a post-approval variation application. The manufacturer shall directly contact the NMRA in the case where the manufacturer intends to change the details of MAH included in the application, which is still under review by the NMRA.
The requirement to transfer a MAH arises from scenarios where the manufacturer decides to divest from the current Marketing Authorization or when the current MA is taken over by another legal entity. The MAH to be transferred is known as the transferor and the company or business partnership or person or legal entity to whom the transfer is to be granted is termed the transferee.
For the MAH transfer process to initiate, the transferor must issue a No Objection Letter (NOL) in agreement with the transfer. If the transferor refuses to issue the NOL, the authority provides 14 days’ timeline to submit the objection against the appointment of transferee (new MAH). If the reason for objection is valid and is concerned with the manufacturer, the authority informs the manufacturer to resolve the objection and proceeds with the transfer process. If the transferor has any commercial conflict with the transferee or manufacturer, the NMRA will proceed with the transfer process as the authority is concerned about device availability in the Sri Lankan market rather than any business obligations between various stakeholders.
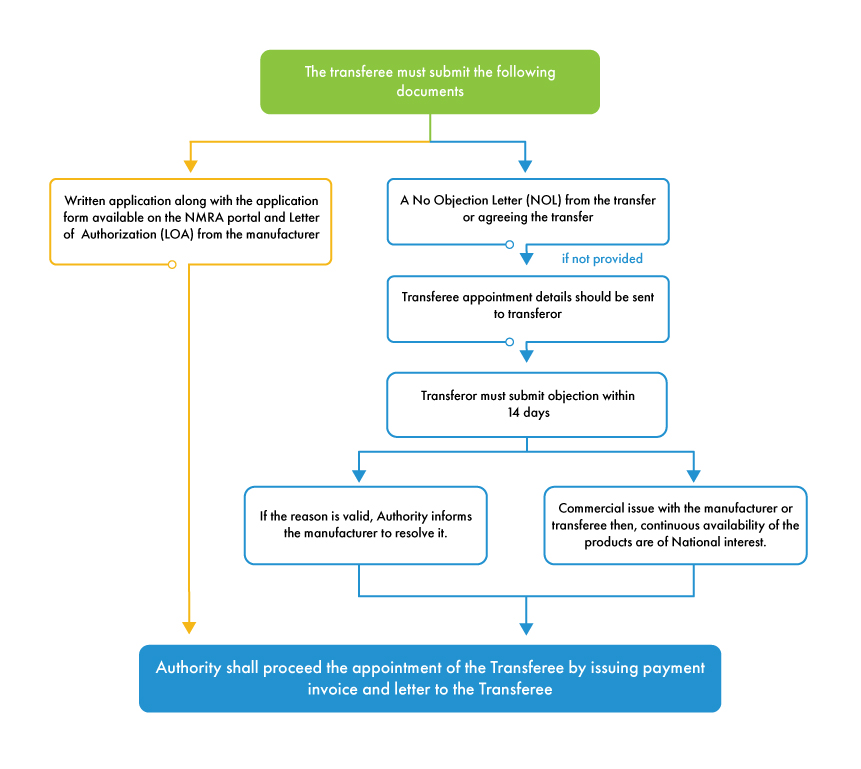
In contrast to other countries, the NMRA regulations are favorable to foreign manufacturers in terms of MAH transfer. NMRA allows MAH transfer with all the required documents unless the objection raised by the transferor is substantial and genuine. It is beneficial for all foreign manufacturers to appoint an independent MAH to avoid any complications in the marketing authorization process of their products in Sri Lanka. Medical device local agent support in Sri Lanka serves a platform for all the foreign manufacturers to appoint and change the MAH seamlessly with proper documentation.
For Local agent /MAH services or for MAH transfer services in Sri Lanka, reach out to a regional Regulatory expert. Stay informed. Stay compliant.









