
In the pharmaceutical industry, compliance with regulatory standards is not just a legal requirement; it's a critical component of patient safety and product quality. One of the most significant aspects of maintaining compliance is the effective management of deviations. Deviations, which can occur at any stage of the pharmaceutical business, from manufacturing to distribution, are departures from established procedures or standards. Proper deviation management is essential for ensuring that pharmaceutical products are safe, effective, and of high quality.
Understanding Deviation Management
Deviation management is a systematic approach to handling unexpected events that can potentially impact product quality or patient safety. It involves the documentation, investigation, and correction of these events, as well as the implementation of measures to prevent their recurrence. This process is integral to a pharmaceutical company's Quality Management System (QMS) and is scrutinized during audits and inspections.
Steps in Deviation Management
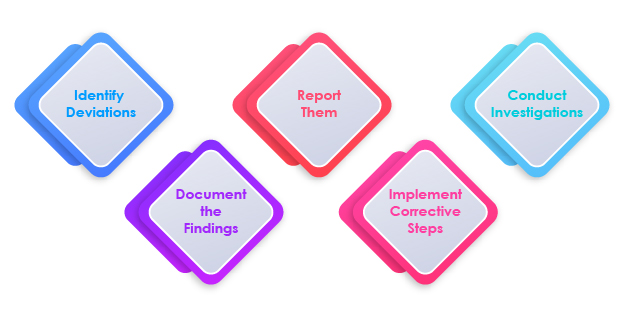
The Role of Deviation Management in Compliance
Guidelines from regulatory bodies such as the FDA and EMA emphasize the importance of a risk-based quality management approach. This approach focuses on critical processes that protect human subjects and ensure the integrity of study data. Deviation management plays a pivotal role in this by identifying, assessing, and mitigating potential risks to study quality.
Impact on Audits and Inspections
During audits and inspections, deviation management is a key area of focus. Auditors examine how deviations are documented, investigated, and resolved. They also evaluate the effectiveness of corrective and preventive actions (CAPAs). A robust deviation management system demonstrates a company's commitment to compliance and continuous improvement, which can positively influence audit outcomes.
Validation of Services and Deviation Management
Validation is the process of ensuring that manufacturing systems and processes consistently produce results meeting predetermined specifications. Deviations observed during validation must be thoroughly investigated to determine their impact on the validation outcome. Proper management of these deviations is crucial for the validation process to be considered reliable and for the systems and processes to be approved for use.
Accelerating Development and Approval
By following regulatory recommendations on deviation management, pharmaceutical companies can accelerate the development and approval of new drugs and biological products. This is because a well-managed deviation system can quickly identify and rectify issues, thereby reducing delays in the development timeline and ensuring that innovations reach patients faster.
Continuous Learning and Improvement
Deviation management is not just about addressing immediate issues; it's also about learning from them. Continuous learning through data collection and analysis is vital for improving the pharmaceutical industry's quality systems. By understanding the root causes of deviations, companies can implement more effective preventive measures, enhancing overall product quality and safety.
Conclusion
Proper deviation management is a cornerstone of pharma compliance, audit, and validation services. It ensures that pharmaceutical companies can maintain the highest standards of product quality and patient safety. By effectively managing deviations, companies comply with regulatory requirements and contribute to the trust and confidence of healthcare providers, patients, and regulatory authorities. As the pharmaceutical industry continues to evolve, the importance of robust deviation management systems will only grow, further underlining their critical role in the lifecycle of pharmaceutical products.









