
The idea of electronic regulatory submissions for pharmaceuticals predates the eCTD tool itself. In the late 1980s, initiatives such as CANDA (Computer-Aided New Drug Application) were explored by the US FDA (Food and Drug Administration) and European agencies. These early efforts sought to enhance efficiency and data access for reviewers.
Enter the eCTD Standard (2003):
The International Conference on Harmonisation (ICH) stepped in 2003 with a game-changer: the eCTD (electronic Common Technical Document) standard. This standardized format was designed to establish a universal language for electronic submissions worldwide.
The Emergence of eCTD Tools:
With the adoption of the eCTD standard, the demand for specialized eCTD tools surged. These tools brought several key benefits:
- Streamlined Content Creation: Users could effortlessly create and organize documents in line with the eCTD structure.
- Robust Validation: eCTD tools ensured submissions met technical specifications, preventing delays caused by formatting errors.
- Efficient Lifecycle Management: Managing revisions, tracking versions, and maintaining regulatory compliance becomes easier.
These innovations revolutionized the regulatory submission process, making it more efficient and reliable.
How Freyr Digital’s Software Automates eCTD Creation
Freyr Digital's advanced software solution, Freyr SUBMIT PRO addresses these challenges by automating key aspects of eCTD creation, thus enhancing efficiency and reducing human error.
Resource Optimization: By automating repetitive tasks, our software frees up valuable human resources. Professionals can focus on more strategic and high-value activities, such as data analysis, regulatory strategy development, and stakeholder engagement. This shift not only enhances productivity but also reduces operational costs, providing a significant return on investment.
Minimized Risk of Human Error: Automation minimizes the risk of human error by ensuring that data is accurately transferred, and documents are correctly formatted. The software's built-in validation checks, and error detection mechanisms further enhance the quality and compliance of regulatory submissions. This reduces the need for rework and helps ensure timely approvals.
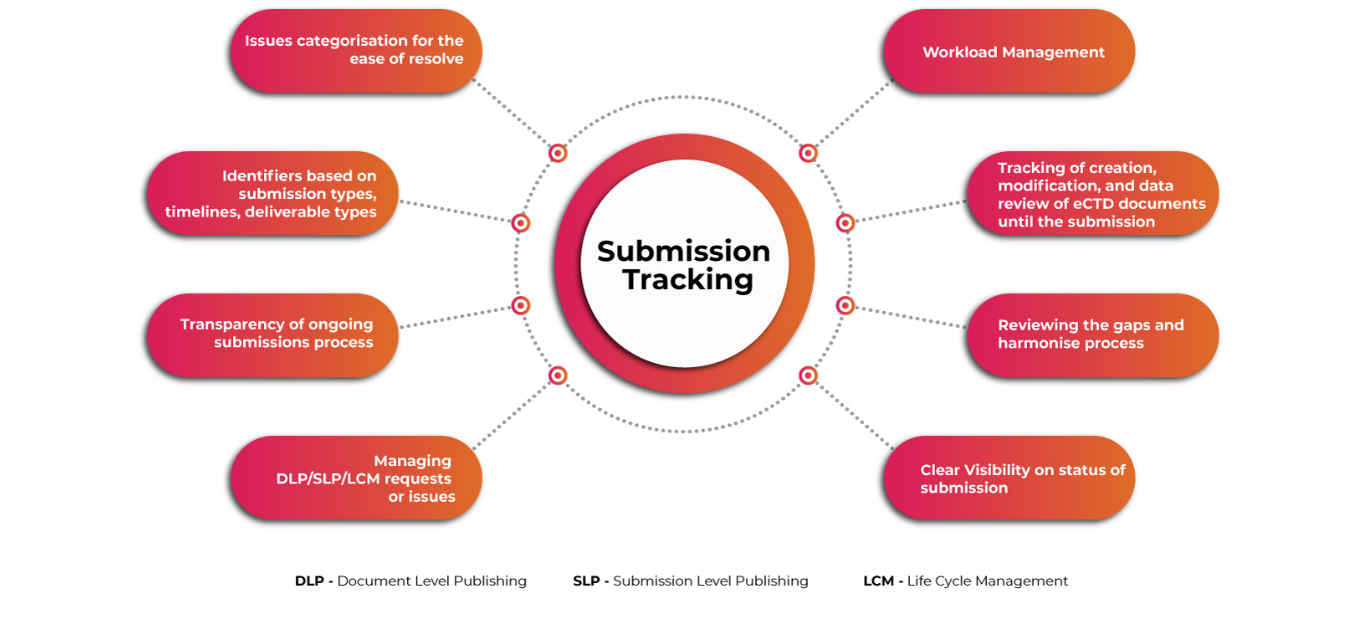
Submissions Tracker for Comprehensive Outlook of eCTD Submissions
A user-friendly automated system for planning & tracking complex global eCTD submission activities, activity workflow management, and storing & managing Regulatory applications and submission information is a must to ensure applicants meet the associated challenges well before time. Getting visibility into submission & publishing operations at a granular level makes the whole process of submissions smoother and faster.
Submission Tracking
The latest upgrade in eCTD
The shift to eCTD 4.0 heralds enhanced efficiency and global standardization in the pharmaceutical drug approval process. By preparing for this transition, your company can seamlessly navigate the change and capitalize on the numerous opportunities this new era offers. While moving to eCTD 4.0 may pose challenges, they are surmountable with dedication and determination from all countries involved. Although significant investment and effort will be necessary, the substantial benefits of eCTD 4.0 make the endeavour truly worthwhile.
Overhaul of Submission Process: eCTD 4.0 promises to revolutionize how pharmaceutical companies submit drug development data to health authorities. It aims to streamline the submission process.
Implementation Timeline: The FDA plans to accept new applications in eCTD 4.0 format starting in 2024. Future phases will address existing v3.2.2 applications and two-way communication.
Forward Compatibility: eCTD 4.0 includes forward compatibility, allowing life cycle and document reuse of v3.2.2 content. This simplifies the conversion of v3.2.2 applications to eCTD 4.0.
Validation Criteria: Specifications for eCTD 4.0 validation criteria are available. These criteria ensure the quality and accuracy of submissions.
Global Compliance: By 2026, regulatory bodies worldwide may require compliance with eCTD 4.0 standards. While currently voluntary, enforcement will begin between 2026 and 2029.
The future of eCTD:
You need to welcome AI and make your regulatory function dynamic as it adds value to your business. Freya Fusion is a cutting-edge, AI-driven, cloud-native Regulatory Information Management (RIM) platform that delivers exceptional performance, security, and scalability, all while maintaining strict GxP compliance. Enhanced with advanced AI/ML and automation features, Freya Fusion excels in the digital realm, providing superior functionality and an outstanding user experience. The AI advancements from Freyr Digital promises Submission Production& tracking modules, encompassing features for seamless compilation, publishing, and validation of regulatory submissions, guaranteeing meticulous compliance with eCTD Guidelines. Staying ahead in the game of regulatory compliance with AI-powered SaaS is sure to benefit your company. Be it with early warning system for Regulatory shifts, streamlined Regulatory document review and analysis or predictive analytics for risk management, Freyr Digital has got you all covered with it. Reach out to hello@freyrdigital.com to discover our AI/ML-driven innovations streamlining regulatory operations in the medical industry.
Stay ahead of the curve with regular updates and technological advancements. Partner with us to streamline your regulatory operations, boost productivity, and elevate submission quality. Request a demo today and experience the perfect blend of expertise and tools. Propel your organization from good to great. Contact us today.









