
Quality Management System (QMS) is an essential component of the medical device industry, ensuring the safety, efficacy, and Regulatory compliance of medical devices throughout their lifecycle. QMS is implemented across all stages of the medical device lifecycle, including the design and development phase, to ensure that the device meets Regulatory and user requirements, and that any potential risks are identified and addressed.
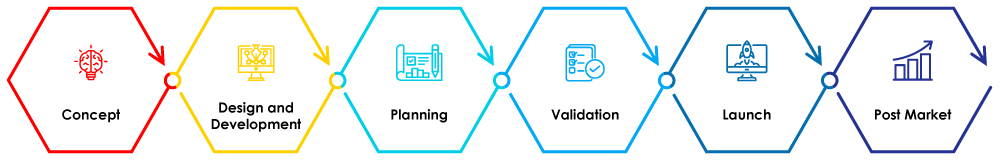
Figure 1-Stages of Medical Device Life Cycle
In this blog, we will discuss the importance of QMS in the design and development phase of the medical device life cycle.
Design and Development Phase in the Medical Device Life Cycle
Design and development phase is one of the most critical stages in the lifecycle of a medical device. During this stage, the device's design is developed and prototypes are created, followed by verification and validation testing as a part of medical device life cycle.
To ensure that the medical device meets the Regulatory requirements, safety, effectiveness, and user expectations, the implementation of a Quality Management System (QMS) is essential at the design and development stage of a medical device’s life cycle.
Documentation is crucial during the design and development phase of medical devices. The QMS ensures that all documentation related to design and development are controlled, managed, and documented.
The Design History File (DHF) is an important file/record, containing all documentation related to the device's design and development. The DHF provides the evidence that the device design meets the Regulatory requirements.
The DHF should contain documentation related to design inputs, design outputs, design reviews, design verification, validation, changes to the design and risk management. Learn more about the DHF here.
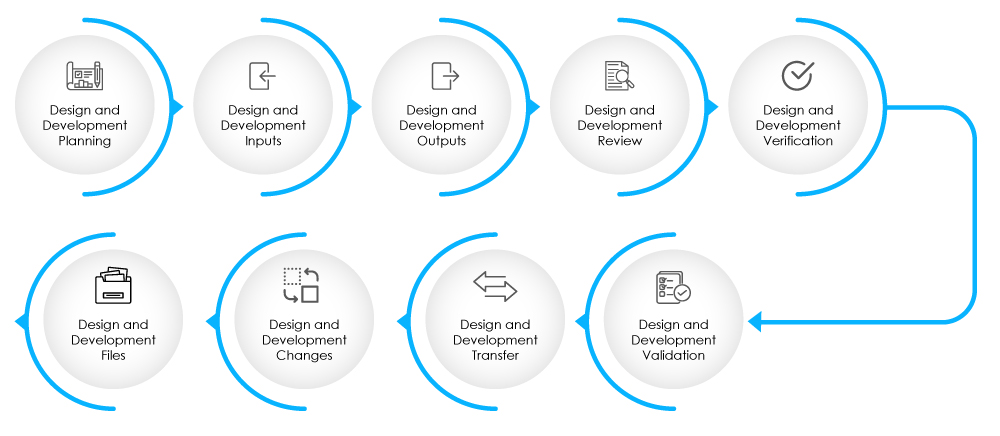
Fig 2-Stages of Design and Development Phase
Best Practices for the Design and Development Stage
- Establish a Structured Approach: Develop a structured approach to DHF development and management that is tailored to the specific needs of your organization. This approach should include clear guidelines, procedures, and workflows for DHF development and management.
- Define and Document Design Inputs: Clearly define and document the design inputs, including the requirements and specifications for the device. This can help ensure that the DHF is comprehensive and complete.
- Manage Design Changes: Implement a robust change management process that includes procedures for documenting, evaluating, and approving design changes. This can help ensure that changes are properly documented and evaluated for their impact on the device's safety and effectiveness.
- Ensure Traceability: Develop a traceability matrix that links the design inputs to the design outputs and ensure that all design and development activities are properly documented and recorded. This can help ensure that the DHF is traceable and that the decision-making process is well-documented.
- Balance Innovation and Compliance: Develop a culture of innovation while ensuring that compliance requirements related to DHF, such as design controls and risk management, are met. This can be achieved by developing procedures and workflows that facilitate innovation while ensuring that Regulatory requirements are met.
- Implement Document Control: Implement document control procedures that ensure the DHF documents are properly controlled, version-controlled, and accessible to authorized personnel. This can help ensure that DHF documents are secure and that changes are properly documented and approved.
- Train the Team: Ensure that the team responsible for DHF development and management is adequately trained on DHF requirements and has the technical expertise to develop the product. This can be achieved through regular training sessions, mentoring, and hiring experienced professionals with the necessary skills and expertise.
By following these best practices, the medical device industry can ensure compliance with the Regulatory requirements, promote the safety and effectiveness of their products, and maintain their competitive edge in the marketplace.
In conclusion, implementing a QMS from the design and development phase is critical for success in the highly regulated medical device industry. By maintaining systematic records and meeting the Regulatory requirements, medical device industry can ensure they are delivering high-quality products and maintaining customer satisfaction.
At Freyr, we offer QMS services to help medical device industry to meet Regulatory requirements across all stages of the medical device life cycle. Get in touch with our QMS and Regulatory experts to know more.









