
When a foreign establishment engages in the production of a medical device imported into the United States (US), it is mandatory to designate a US agent for that establishment. The US agent serves as the point of contact for any US Food and Drug Administration (US FDA) communication related to the foreign registered facility. Since this communication can be time-sensitive, it is imperative for the US agent to possess a comprehensive understanding of the US FDA regulations and be capable of advising the foreign establishment on compliance with the US FDA’s medical device requirements. Moreover, having an independent US agent helps prevent potential conflicts of interest that may arise when a business partner is designated as the US agent. In this blog, we will delve into the details of the registration of a foreign establishment with the US FDA.
What is Establishment Registration?
Annual registration with the US FDA is mandated for Contract Manufacturers, Initial Importers, Repackers/Relabelers, Specification Developers, etc., of establishments engaged in the manufacturing and distribution of medical devices intended for the U.S. market, a procedure referred to as Establishment Registration (Title 21 CFR Part 807).
Who All Need to Apply for Establishment Registration?
Owners of facilities who are engaged in the manufacturing and distribution of medical devices intended for use in the US must complete an annual registration with the US FDA. The types of owners who can apply for establishment registration are:
- Owners of Domestic Establishments
- Owners of Foreign Establishments
- Contract Manufacturers
- Initial Importers
- Repackers/Relabelers
- Specification Developers
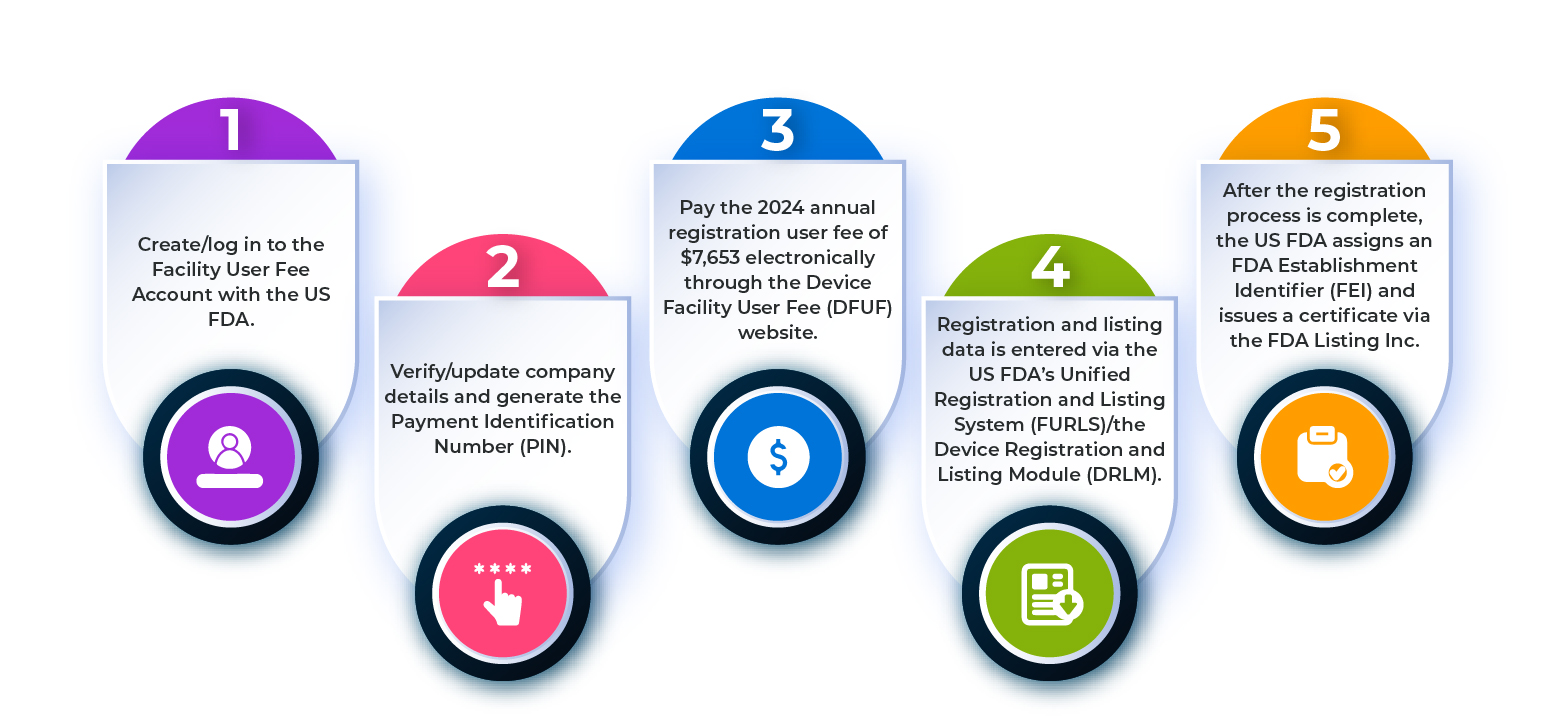
What are the Steps Involved in the Establishment Registration Process?
To register a medical device establishment with the US FDA, you need to follow the steps outlined in the flowchart below.
Figure 1: Steps in the Establishment Registration Process
What are the Foreign Establishment Registration and Listing Requirements?
Understanding the significance of choosing an independent, expert US agent will help a foreign establishment prioritize the avoidance of any conflict of interest, which is paramount for the establishments as they navigate the intricate realm of US FDA compliance. Foreign establishments must bolster their understanding of prerequisites for achieving compliance, foster strong partnerships with US agents, and set the stage for a smooth registration process. The table below outlines the registration and listing requirements based on a foreign establishment’s activities. It also indicates which activities incur an establishment registration fee.
Table 1: Registration and Listing Requirements Based on a Foreign Establishment’s Activities
| Activity | Register | List | Pay Fee | |
| Contract Manufacturer (including Contract Packagers) | YES 807.40 (a) | YES 807.40 (a) | YES | |
| Contract Sterilizer | YES 807.40 (a) | YES 807.40 (a) | YES | |
| Custom Device Manufacturers | YES 807.20 (a) (2) | YES 807.20 (a) (2) | YES | |
| Device Being Investigated under Investigational Device Exemption (IDE) | NO 812.1 (a) | NO 812.1(a),807.40 (c) | NO | |
| Foreign Exporter of Devices Located in a Foreign Country | YES 807.40 (a) | YES 807.40 (a) | YES | |
| Foreign Manufacturers (including Kit Assemblers) | YES 807.40 (a) | YES 807.40 (a) | YES | |
| Maintains Complaint Files as Required under 21 CFR 820.198 | YES | YES | YES | |
| Manufacturer of Accessories that are Packaged or Labeled for Commercial Distribution for Health-related Purposes to an End User | YES 807.20 (a) (5) | YES 807.20 (a) (5) | YES | |
| Manufacturer of Components that are Distributed Only to a Finished Device Manufacturer | NO 807.65 (a) | NO | NO | |
| Relabelers or Repackers | YES 807.20 (a) (3) | YES 807.20 (a) (3) | YES | |
| Remanufacturer | YES | YES | YES | |
| Reprocessor of Single-use device | YES 807.20 (a) | YES 807.20 (a) | YES | |
| Specification Developers | YES | YES | YES | |
Establishment registration with the US FDA is an essential requirement for foreign establishments involved in producing, selling, repackaging, or importing medical devices in the US. This registration ensures compliance with the US FDA’s strict regulations and standards. Freyr provides valuable support to establishments seeking expert guidance during the establishment registration process. Leveraging their expertise and understanding of the US FDA’s regulations, Freyr assists foreign establishments in meeting compliance requirements, ultimately leading to a smoother and more efficient entry into the US medical device market. To know more about the establishment registration process with the US FDA, reach out to us today. Stay informed! Stay compliant!









