SOP Writing and SOP Review Services - Overview
The Life Sciences industry is moving towards procedural standardization, risk-based methodology, Quality by Design (QbD), and a common control framework. However, Standard Operating Procedures (SOP) review and SOP writing services may sometimes require organizations to invest time and money to research global legislation and update the systems perennially. In such scenarios, organizations look for expert SOP writing services and SOP review services. Our SOP writing and SOP review services help organizations streamline their processes and ensure that all SOP preparation activities are aligned with the latest Regulatory expectations.
Freyr provides SOP authoring services for all types of pharmaceutical practices, research and development, Annual Product Quality Review (APQR), information technology, Regulatory Affairs, quality management review, clinical research, and all categories of pharmaceutical SOPs. Our SOP writing services are tailored to meet the unique needs of each client, ensuring that SOP preparation is thorough and compliant with industry standards.
With a comprehensive knowledge of new SOP writing/SOP review or to amend/standardize existing ones, our team offers end-to-end SOP writing services and SOP review services that span across:
| SOP Writing Services | SOP Review Services | SOP Integration Services |
|---|---|---|
| Process designing and establishment by writing SOPs for a new management system. SOP writing for the existing management system (for new and targeted processes). | Review existing SOPs for accuracy and provide gap analysis reports. Review existing SOPs for accuracy and provide a remediation report. SOP reconciliation services (Standardization, Rationalization, and Optimization). | For Mergers and Acquisitions. |
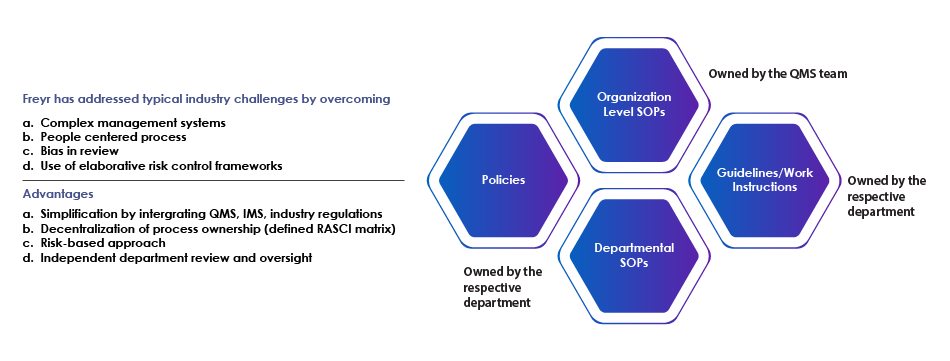
| Freyr offers an integrated process of multiple management systems (ISO 9001, ISO 27001 with USFDA, EMEA, and MHLW). | ||
SOP Writing and SOP Review Services
SOP Gap Analysis
Freyr has been providing SOP writing services and SOP review services for the pharma, healthcare, and biotechnology industries. In the last five (05) years, Freyr’s Compliance, Audit & Validation Centre of Excellence (CoE) has created/reviewed/harmonized five thousand five hundred (5500) + SOPs for its customers and continues to support them. Our SOP writing services have been instrumental in helping organizations achieve Regulatory milestones.
Following the risk-based approach and common control framework methodologies, we support organizations with integrated SOPs catering to all Regulatory requirements. Our QMS remediation experts can establish process architecture by integration of multiple regulations for unified management systems (ISO 9001, ISO 27001, GLP – 17025, Medical Laboratories – 15189, CAP with USFDA, EMEA, MHLW, WHO, GxP, and ICH). SOP writing and SOP review services are essential in maintaining the integrity and consistency of these systems.

- Process designing
- Establishing process architecture
- Risk-based approach
- Common control framework
- Integration of multiple regulations for unified management systems
Additionally, Freyr has provided audit and audit readiness support (including SOP writing) for twenty (20) + large/medium/small pharma companies with global Health Authorities (HAs), including the US FDA, MHRA, EMA, CDSCO, ANVISA, SFDA, HALMED, NPRA, HSA, and Health Canada. Our SOP writing services and SOP review services ensure organizations are always prepared for inspections and audits.
Process Approach for Updating and Creating SOPs
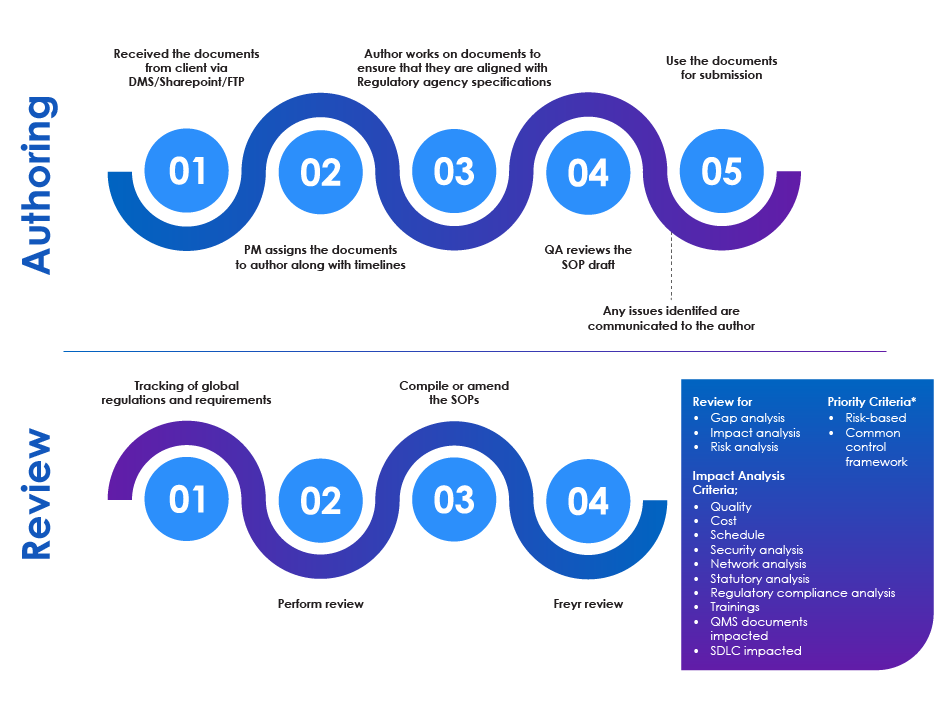
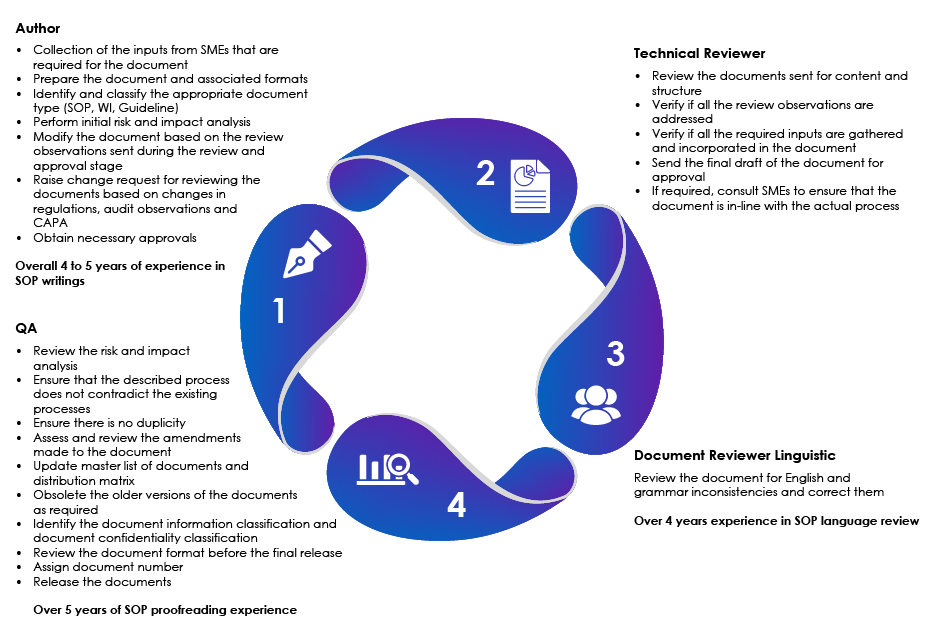
SOP Authoring and Review
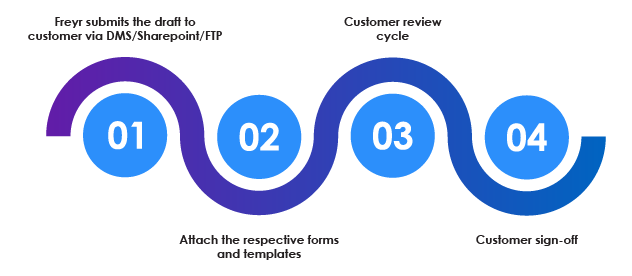
Customer Review and Sign Off
Overview of the SOP Writing Department
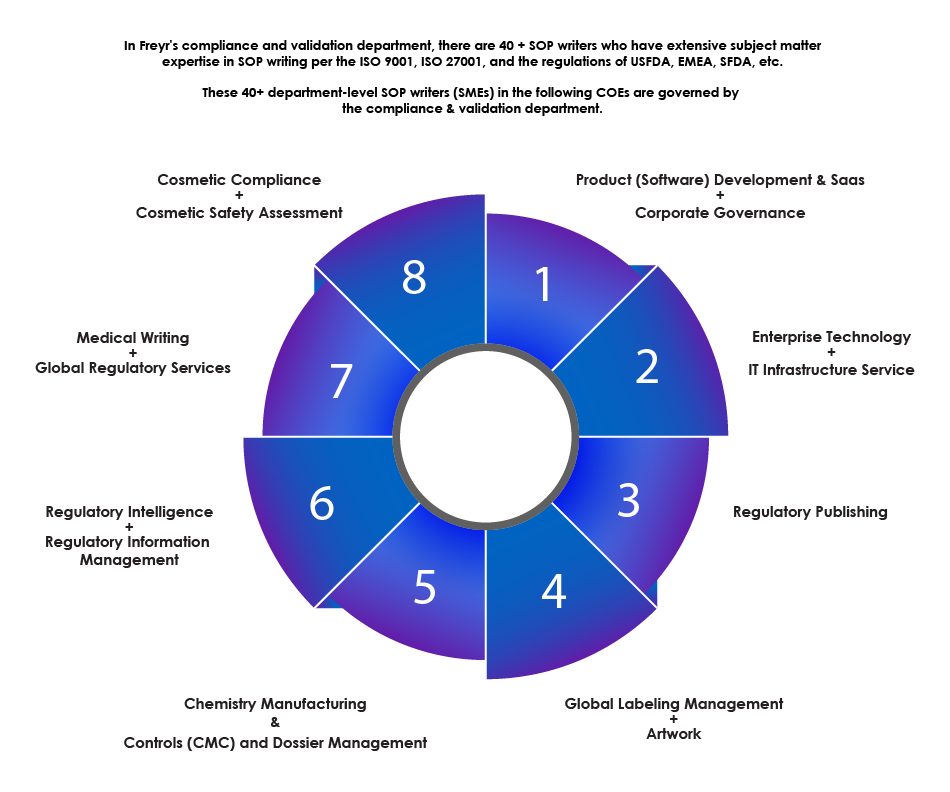
Freyr’s expertise in SOP writing
SOP Review Services
While expanding businesses to newer markets, life sciences organizations are required to develop new SOPs based on the regional Regulatory requirements or must update/upgrade the existing processes to align them with new demands. But before SOP preparation, the right approach is to identify the gaps between processes and remediate them accordingly. SOP writing and SOP review services are designed to support organizations at every stage of SOP preparation, from initial drafting to final approval.
Freyr is experienced in a range of projects on SOP review services. The simplest part of the service offering is to review existing SOPs for accuracy and provide gap analysis reports to complex projects, which involve SOP review and remediation, resulting in SOP standardization, rationalization, or SOP optimization. SOP rationalization is a key element of our SOP review services, ensuring each document adds value and supports operational excellence.
SOP Standardization
As the business grows, the standards to be followed or adhered to will also increase. There will be times when a customer has several standards and regulations to follow, which may include SOPs for safety in the pharmaceutical industry. In such scenarios, maintaining and following different streams of SOPs for each standard will double up the procedural complexities for companies. The need of the hour is to standardize the SOPs. SOP writing services play a crucial role in the standardization process, ensuring uniformity and compliance.
SOP Rationalization
At times, organizations create SOPs to let go of the audit process. But there is no use implementing these. Either they are not followed by employees, or they could make processes complex if they are integrated. To avoid such situations, it is necessary to rationalize the SOPs that bring some value to the organization. SOP rationalization, as part of our SOP review, helps streamline documentation and remove unnecessary complexity.
Freyr provides SOP review services and rationalizes them in addition to adhering to applicable regulations and assists organizations succeed in SOP compliance audits, thus adding real value to the company. SOP writing and SOP review services are vital components in maintaining effective quality management systems.
SOP Reconciliation and SOP Optimization
Though there are no guidelines, SOPs must be reviewed at defined and periodic intervals as a general practice. With different formats and versions accumulated, there is always a chance for overlapping and duplication. During such times, organizations must reconcile their SOPs, while continually improving processes to support their growth and business. SOP writing services and SOP review services are essential in this ongoing process.
With knowledge of SOPs and quality systems, Freyr assists organizations in reconciling and optimizing their SOPs periodically. Our expert compliance team can monitor and carry out incremental updates.
SOP Integration
Mergers and acquisitions are an integral part of the current business process. But when they take place, bringing together and managing the procedures, standards, SOPs, and quality systems of both organizations could be grueling. In addition to aligning with the existing standards, setting up SOPs for the newly formed organization can be even more hectic, especially with short TATs.
With an in-house compliance team, Freyr offers end-to-end SOP integration services to analyze the gaps between both organizations’ systems, standardize existing systems, and design new SOPs when needed. Our experts extend system integration support for designing seamless processes and workflows.
- Proposing cost-effective process options without compromising the objective of product quality, Regulatory compliance, patient safety, data integrity, and security
- Integrated process models
- Proven practicable validation and qualification strategies
- Quick turnaround time
SOP writing and SOP review services from Freyr ensure that your organization benefits from best-in-class SOP writing services, SOP review services, and SOP rationalization, supporting every phase of SOP preparation and compliance management.








