
The European Union's (EU) Medical Device Regulation (MDR) has been making news for a while now. The MDR has replaced the Medical Device Directive (MDD) and Active Implantable Device Directive (AIMDD). Initially, the entire transition was set out to be in complete effect by May 2020; however, due to the emergence of the COVID-19 pandemic, the implementation was pushed further to May 26, 2021. In this timeline, by May 26, 2024, all the MDD certificates will become void, and the device manufacturers will be required to conform with the EU MDR. Further, the MDD devices lawfully placed on the market pursuant to Directives 90/385/EEC and 93/42/EEC prior to May 26, 2020, and devices placed on the market from May 26, 2020, by virtue of a certificate will continue to be made available in the market until May 27, 2025. The timelines are depicted below –
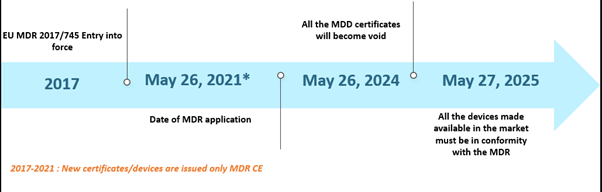
The EU MDR Past Scenario Timelines
However, the limited capacity of the Notified Bodies (NBs) and the unpreparedness of manufacturers laid some challenges in the implementation of the MDR as per the given timeline. As of October 2022, in total, there are thirty-eight (38) Notified Bodies (NBs), and these NBs received about 8120 applications for the EU MDR certification, out of which 1990 certificates have been issued. As per their estimations with the initial timeline, only 7000 certificates could be processed, and this further led to the timeline extension. In addition, one of the other probable reasons for the extension was to ensure the continued availability of safe medical devices whose certificates have already expired or are due to expire before May 26, 2024. The current scenario for the extended timeline is depicted below-
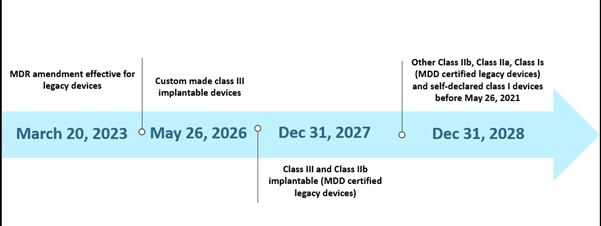
The EU MDR Past Scenario Timelines
The new extension is applicable to legacy devices fulfilling article 120 (3e) with a valid MDD CE or derogation as on Mar 20, 2023, and will remain in the market along with the MDR CE-marked devices. By May 26, 2024, manufacturers of legacy devices should have an implemented QMS in place and lodged an application with an MDR-designated NB for conformity assessment, and by Sep 26, 2024, manufacturers of legacy devices should have an agreement with an MDR-designated NB.
Now let’s look at the impact the manufacturers might have with this extension.
Opportunities the manufacturers have with this extension:
- Extended market access for the MDD/AIMDD-certified device manufacturers who have already taken the MDR compliance initiatives.
- The MDR-certified manufacturers whose MDD/AIMDD CE certificates have not been revoked are permitted to place legacy devices on the market until the end of the transition period in addition to their MDR-compliant devices.
- Manufacturers who have a national derogation as of March 20, 2023, can be benefitted from the transitional period.
- The extension period buys more time for a better understanding of the rules and regulations, which helps to streamline the process and achieve MDR compliance.
Challenges that could arise for manufacturers with this extension:
- There is no market benefit for legacy device manufacturers who did not want to comply with the MDR.
- The MDR’s extension may cause certification processes to drag out and delay product launches, which is a direct result of the backlog of the reviews by NBs.
What Actions Should Manufacturers Take?
- It is imperative that the manufacturers determine the MDR risk class of their medical device to promptly identify the appropriate transitional timeline as per the amended MDR regulations.
- To ensure compliance with the MDR regulations, it is crucial to identify and initiate communication with the MDR-designated NBs possessing the specific competency required for the classification of your medical device.
- It is critical to perform a comprehensive gap assessment for your medical device certified under the MDD/AIMDD, identify and address any non-conformances with the MDR regulations and ensure timely compliance.
It is essential for manufacturers to take immediate action to ensure compliance with the MDR. The extended timeline offers some opportunities for manufacturers to achieve MDR compliance, but it also presents challenges, such as delayed certification processes and the cost of compliance. To navigate these challenges and capitalize on the opportunities, let our team of professionals assist you through the MDR compliance process and ensure your success in this challenging Regulatory environment. Book an appointment with us today to learn more about how we can help you achieve the MDR compliance and stay ahead of the curve. Stay informed. Stay compliant.









