
In the fast-paced and heavily regulated world of life sciences, the Medical, Legal, and Regulatory (MLR) review process is crucial for ensuring that all promotional materials, educational content, and communications are accurate, compliant, and ethically sound. However, this process can be time-consuming and complex, often leading to delays and inefficiencies. This blog explores strategies to make the MLR review and approval process more efficient and effective, ultimately improving the speed and quality of healthcare communications.
Understanding the MLR Review Process
The MLR review process involves a multidisciplinary team of medical professionals, legal advisors, and regulatory affairs specialists. Each member of the team plays a vital role in scrutinizing content to ensure it meets the highest standards of accuracy, compliance, and ethics. The process typically includes the following steps:
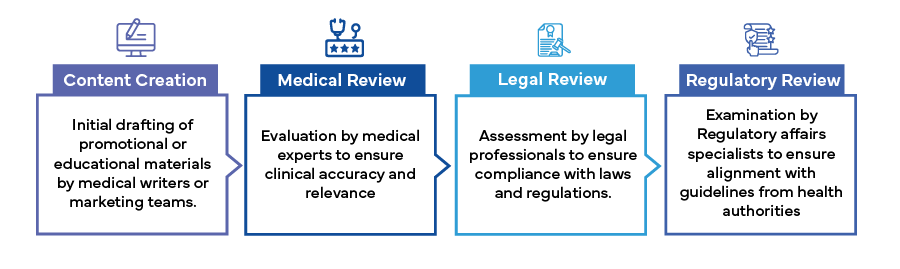
Challenges in the MLR Review Process
Despite its importance, the MLR review process faces several challenges:
- Time-Consuming: The review process can be lengthy due to multiple rounds of feedback and revisions.
- Complex Coordination: Coordinating input from various departments and experts can be challenging.
- Regulatory Changes: Keeping up with ever-changing regulations requires constant vigilance and adaptability.
- Consistency: Ensuring consistency in messaging and compliance across all materials is difficult.
Strategies for Enhancing Efficiency and Effectiveness
Implementing a Centralized Review System
A centralized review system can streamline the MLR process by providing a single platform for all stakeholders to access, review, and approve materials. This system should include version control, real-time collaboration tools, and automated workflows to reduce manual effort and improve transparency.
Leveraging Technology and AI
Advancements in technology, such as artificial intelligence (AI) and machine learning, can significantly enhance the efficiency of the MLR review process. AI-powered tools can automate repetitive tasks like initial content checks for compliance and consistency, freeing up human reviewers to focus on more complex evaluations.
Establishing Clear Guidelines and Templates
Providing clear guidelines and standardized templates for content creation can help ensure that all materials meet the required standards from the outset. This reduces the need for extensive revisions and speeds up the approval process.
Training and Continuous Education
Regular training and continuous education for MLR team members are essential to keep them updated on the latest regulations, best practices, and technological tools. This ensures that the team can effectively manage the review process and stay compliant with current standards.
Enhancing Communication and Collaboration
Effective communication and collaboration among MLR team members are crucial for a smooth review process. Regular meetings, clear communication channels, and collaborative tools can help ensure that all team members are on the same page and can provide timely feedback.
Regular Audits and Feedback Loops
Conducting regular audits of the MLR review process and establishing feedback loops can help identify areas for improvement. This continuous improvement approach ensures that the process remains efficient and effective over time.
The Role of Freyr Solutions
At Freyr Solutions, we specialize in providing regulatory services that help life sciences companies streamline their MLR review processes. Our team of experienced professionals offers a comprehensive suite of services, including:
- Developing and implementing centralized review systems.
- Leveraging AI-powered tools for efficient content checks.
- Providing clear guidelines and standardized templates.
- Offering training and continuous education programs.
- Enhancing communication and collaboration among MLR teams.
- Conducting regular audits and providing actionable feedback.
Conclusion
Making the MLR review and approval process more efficient and effective is essential for the timely and compliant dissemination of healthcare information. By implementing a centralized review system, leveraging technology, establishing clear guidelines, and enhancing communication and collaboration, life sciences companies can overcome the challenges of the MLR review process. Freyr Solutions is committed to supporting companies in this endeavor, ensuring their communications are accurate, compliant, and ethically sound.









