
Clinical research is a cornerstone of medical advancement, providing the necessary evidence to support the safety and efficacy of new treatments and interventions. At the heart of this research lies the clinical trial protocol, a document that outlines the objectives, design, methodology, and organization of a clinical trial. The development of a robust clinical trial protocol is critical, and peer review plays a vital role in ensuring its scientific validity and ethical integrity.
Understanding Clinical Trial Protocols
A clinical trial protocol is not just a blueprint for conducting research; it is a contract between the investigators, sponsors, and regulatory bodies. It details every aspect of the trial, from the hypothesis and objectives to the eligibility criteria, procedures, and statistical analysis plan. The protocol must address safety issues, ethical considerations, and the overall feasibility of the study.
The Role of Peer Review
Peer review is an essential process in which experts in the field critically evaluate the clinical trial protocol before the study commences. This scrutiny serves several key purposes:
- Ensuring Scientific Rigor: Peer reviewers assess the scientific rationale behind the trial, the clarity of the research question, and the appropriateness of the methodology. They ensure that the study design can answer the research question and that the statistical analysis plan is adequate to interpret the results.
- Protecting Participant Safety: Reviewers evaluate the potential risks and benefits to participants, ensuring that safety measures are in place and that the trial adheres to ethical standards. They confirm that the rights, safety, and well-being of trial subjects are prioritized over the interests of science and society.
- Enhancing Credibility: A protocol that has undergone rigorous peer review gains credibility among stakeholders, including regulatory authorities, ethics committees, and potential participants. It demonstrates a commitment to quality and integrity in the research process.
- Facilitating Regulatory Approval: Regulatory bodies often require evidence of peer review as part of the clinical trial application process. A well-reviewed protocol is more likely to meet the stringent requirements for trial approval and subsequent drug or device registration.
- Promoting Collaboration: Peer review encourages collaboration and knowledge sharing among researchers. It can lead to the identification of potential issues and the incorporation of suggestions that strengthen the protocol.
The Peer Review Process
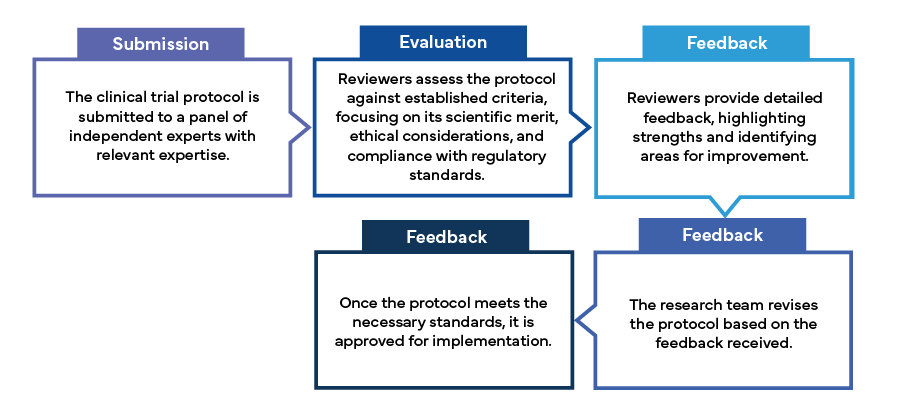
The peer review process typically involves the following steps:
Conclusion
Peer review is a cornerstone of clinical trial protocol development, ensuring scientific rigor, participant safety, and regulatory compliance. Partnering with Freyr Solutions for peer review services offers additional benefits, including access to a team of experienced professionals with diverse expertise in regulatory affairs, medical writing, and clinical research. Freyr's collaborative approach and commitment to quality assurance can help streamline the peer review process, expedite protocol approval, and enhance the overall success of clinical trials.









