In the global pharmaceutical industry, the accurate translation of Regulatory documents safeguards medicinal products’ safety and efficacy standards across different regions. It facilitates successful submissions and market entries. However, translating these documents is accompanied by challenges such as complex terminologies and strict adherence to guidelines. We shall explore best practices and common pitfalls in translating Regulatory documents, emphasizing the importance of precision and consistency.
The Complexity of Regulatory Translations
Translating Regulatory documents involves more than just converting text from one language to another. It requires a deep understanding of Regulatory language, cultural nuances, and the specific requirements of different health authorities. Errors and inconsistencies in translation can lead to misinterpretations, Regulatory setbacks, and even jeopardize patient safety.
Common Pitfalls in Regulatory Translations
Literal Translations
Literal translations can lead to misunderstandings and loss of meaning. Translators must find a balance between staying true to the original text and making the translation comprehensible and relevant to the target audience.
Inconsistent Terminology
Inconsistent use of terminology can create confusion and misinterpretation. It is essential to maintain uniformity in the translation of critical terms across all documents to ensure clarity and accuracy.
Lack of Context
Translating without understanding the context can result in errors. Translators must have a thorough understanding of the subject matter and the Regulatory environment to accurately convey the intended meaning.
Ignoring Regulatory Nuances
Each Regulatory body has its own set of guidelines and requirements. Ignoring these nuances can lead to non-compliance and delays in the approval process. Translators must be well-versed in the specific regulations of the target region.
Best Practices for Translating Regulatory Documents
Use Specialized Translators
Specialized translators with expertise in Regulatory language are crucial. They possess the necessary knowledge to accurately translate complex medical and technical terms, ensuring that the translated documents are precise and compliant with Regulatory standards.
Maintain Consistent Terminology
Consistency in terminology is vital in Regulatory translations. Establishing a comprehensive glossary of terms helps maintain uniformity across all documents, reducing the risk of misinterpretation and ensuring that the translated content aligns with the original intent.
Implement Rigorous Quality Control
Quality control is a critical component of the translation process. This involves multiple levels of review, including proofreading, editing, and back-translation, to ensure the highest level of accuracy. Quality control measures help catch and correct errors, ensuring that the final document is error-free and compliant with Regulatory requirements.
Adhere to Regulatory Guidelines
Understanding and adhering to the specific guidelines of different Regulatory bodies is essential. Translators must be familiar with the requirements of authorities such as the US FDA, EMA, PMDA, and NMPA to ensure that the translated documents meet all necessary standards.
Table: Best Practices vs. Common Pitfalls in Translation
| Common Pitfalls | Solutions/ Best Practices |
|---|---|
| Literal translations errors | Use of specialized translators |
| Inconsistent terminology | Consistent terminology |
| Lack of context in translations | Rigorous quality control |
| Ignoring Regulatory nuances | Adherence to Regulatory guidelines |
How would Regulatory partners assist pharma firms?
Regulatory partners play a vital role in ensuring accurate and compliant translations. They offer expert translation services, implement best practices, and provide robust quality control measures. By leveraging the expertise of Regulatory partners, pharmaceutical companies can streamline their translation processes, reduce risks, and enhance the accuracy and reliability of their Regulatory documents.
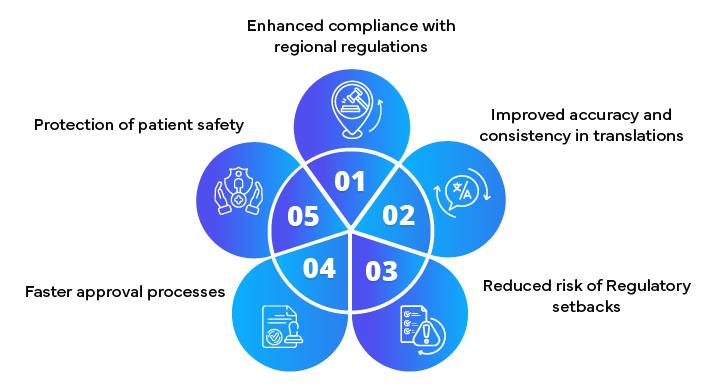
Importance of Accurate Translations
Summary
Accurate translation of Regulatory documents is crucial for global compliance in the pharmaceutical industry. By following best practices and avoiding common pitfalls, companies can ensure that their documents meet the highest standards of accuracy and Regulatory compliance. Partnering with Regulatory experts further enhances the quality and reliability of translations, facilitating successful submissions and market entries.