As we now move toward the end of 2024, Regulatory professionals find themselves at the forefront of exciting innovations and challenging trends that are reshaping how medicinal drug products, medical devices, and biotechnology products are developed, approved, and monitored. This blog explores the cutting-edge developments in Regulatory affairs and how they're transforming the industry.
Furthermore, the rapid advancement of science and technology, coupled with changing patient expectations and global health challenges, has created a complex Regulatory environment. Traditional approaches to Regulatory affairs are struggling to keep pace with innovations like artificial intelligence, real-world evidence, and personalized medicine. Regulatory bodies and life sciences companies alike are grappling with how to ensure patient safety and product efficacy while fostering innovation and speeding up market access.
Novel RA Trends and Regulatory Innovations:
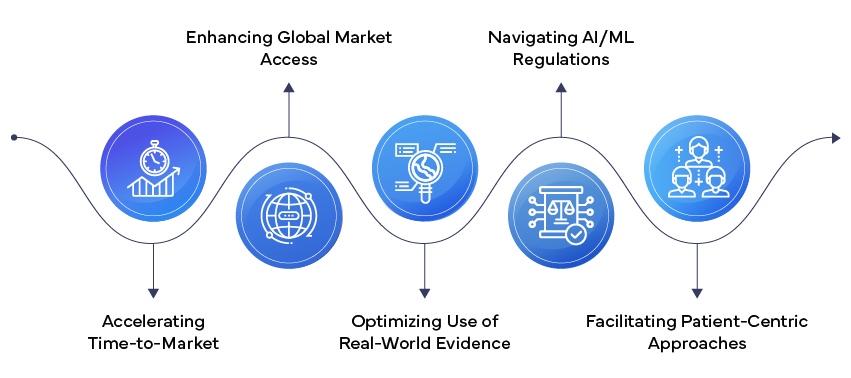
- Artificial Intelligence and Machine Learning: AI and ML are revolutionizing Regulatory affairs, from predictive modeling for clinical trial design to automated adverse event detection. The FDA's recently proposed framework for AI/ML-based Software as a Medical Device (SaMD) signals a shift towards more adaptive Regulatory approaches for these rapidly evolving technologies.
- Real-World Evidence (RWE) Integration: Regulatory agencies are increasingly accepting RWE to support Regulatory decision-making. This trend is enabling faster approvals and more comprehensive post-market surveillance. The 21st Century Cures Act in the US has been a significant driver in promoting the use of RWE in Regulatory submissions.
- Patient-Centric Regulatory Approaches: There's a growing emphasis on incorporating patient perspectives throughout the Regulatory process. Patient-reported outcomes and patient preference information are becoming integral to Regulatory submissions, reflecting a shift towards more patient-centric drug development and approval processes.
- Accelerated Approval Pathways: Regulatory bodies are introducing and refining accelerated approval pathways to speed up access to innovative therapies, especially for rare diseases and unmet medical needs. The FDA's Breakthrough Therapy designation and the EMA's PRIME (PRIority MEdicines) scheme are examples of this trend.
- Global Regulatory Harmonization: Initiatives like the International Council for Harmonisation (ICH) are driving greater alignment in Regulatory requirements across regions, facilitating simultaneous global submissions and reducing Regulatory burden.
- Digital Health Regulation: The rise of digital health technologies, including mobile medical apps and wearable devices, is prompting Regulatory bodies to develop new frameworks for assessing these products. The FDA's Digital Health Center of Excellence is at the forefront of this evolving Regulatory landscape.
Table: Comparison of Traditional vs. Innovative Regulatory Approaches
| Aspect | Traditional Approach | Innovative Approach |
|---|---|---|
| Data Sources | Primarily clinical trials | Clinical trials + Real-world evidence |
| Review Process | Linear, stepwise | Adaptive, continuous |
| Patient Input | Limited | Integral throughout development |
| Technology Use | Basic data analysis | AI/ML-powered analytics |
| Approval Pathways | Standard pathways | Multiple accelerated options |
| Global Strategy | Region-specific | Harmonized global approach |
Role of Regulatory Experts:
In this rapidly evolving landscape, Regulatory experts play a crucial role in helping life sciences companies navigate complex challenges and leverage new opportunities.
Key services provided by them include:
- Strategic Regulatory planning aligned with innovative development approaches
- Expertise in leveraging AI/ML for Regulatory intelligence and submissions
- Guidance on integrating RWE into Regulatory strategies
- Support in patient engagement and incorporating patient perspectives
- Navigation of accelerated approval pathways and global harmonization initiatives
- Expertise in digital health regulations and emerging technology frameworks
Advantages of having a Regulatory Expert
The Regulatory affairs landscape is undergoing a profound transformation, driven by technological advancements, patient-centric approaches, and the need for more efficient drug development processes. As the industry embraces these novel trends and innovations, the role of Regulatory professionals becomes increasingly critical. By staying ahead of these trends and leveraging the expertise of Regulatory partners, life sciences companies can navigate this complex landscape more effectively, bringing innovative therapies to patients faster while maintaining the highest standards of safety and efficacy. The future of Regulatory affairs is not just about compliance; it's about driving innovation and improving global health outcomes. Click here to learn more about Regulations and compliance practices.