The pharmaceutical industry today faces unprecedented opportunities for global expansion. However, navigating the complex landscape of international Regulatory requirements can be daunting. This blog explores how strategic Marketing Authorization Holder (MAH) operations can be the key to unlocking your company's global potential, all while ensuring Regulatory compliance and patient safety.
The Challenge:
As life sciences companies look to expand their reach, they often encounter a maze of Regulatory hurdles. Each country has its own set of rules, procedures, and cultural nuances that can make global expansion seem like an insurmountable task. The need for local representation, understanding of market-specific requirements, and management of pharmacovigilance activities across borders can quickly overwhelm even the most seasoned pharmaceutical companies.
Strategic MAH Operations:
Marketing Authorization Holders play a pivotal role in the lifecycle of a medicinal product, from initial authorization to ongoing safety monitoring. By strategically leveraging MAH operations, companies can:
- Streamline Market Entry: MAHs can act as your local representative, facilitating smoother interactions with Regulatory authorities and expediting the approval process.
- Ensure Regulatory Compliance: With in-depth knowledge of local regulations, MAHs help maintain compliance across multiple jurisdictions, reducing the risk of costly violations.
- Manage Pharmacovigilance: MAHs oversee crucial safety monitoring activities, ensuring that your products meet the highest standards of patient safety in every market.
- Navigate Cultural Differences: Local MAHs bring invaluable insights into market-specific preferences and practices, helping tailor your approach for maximum impact.
- Optimize Resource Allocation: By partnering with established MAHs, companies can avoid the need for extensive local infrastructure, allowing for more efficient use of resources.
Table: Key Responsibilities of Marketing Authorization Holders
| Responsibility | Description |
|---|---|
| Regulatory Submissions | Prepare and submit marketing authorization applications |
| Safety Monitoring | Implement and maintain pharmacovigilance systems |
| Quality Assurance | Ensure product quality meets local standards |
| Communication | Liaise with health authorities and manage product information |
| Compliance | Stay updated on and adhere to local Regulatory requirements |
Regulatory Partners: Role in MAH Projects
Navigating the complexities of global MAH operations requires expertise and resources that many companies may not have in-house. This is where Regulatory partners come into play.
They offer:
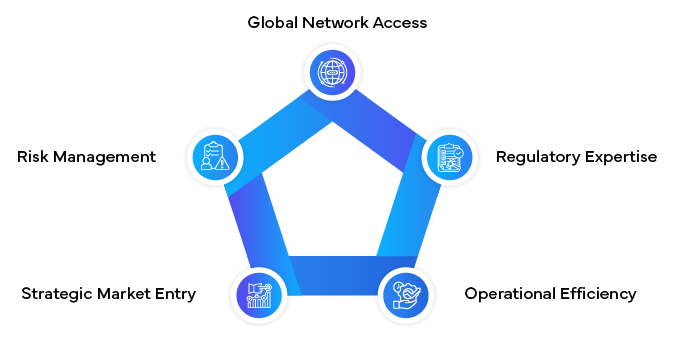
- Extensive Network: Access to a global network of MAHs, simplifying expansion into multiple markets simultaneously.
- Regulatory Expertise: In-depth knowledge of diverse Regulatory landscapes, ensuring compliance across borders.
- Operational Support: Management of day-to-day MAH activities, allowing you to focus on core business functions.
- Strategic Guidance: Insights into market entry strategies and Regulatory pathways tailored to your products and goals.
- Risk Mitigation: Proactive identification and management of potential Regulatory risks.
Role of Regulatory Partners in Global MAH Operations
Summary:
In an era where global expansion is both an opportunity and a necessity for life sciences companies, strategic MAH operations have become indispensable. By leveraging the expertise of MAHs and Regulatory partners, pharmaceutical companies can navigate the complexities of international markets with confidence. This approach not only ensures Regulatory compliance but also optimizes resource allocation, accelerates market entry, and ultimately contributes to improved patient access to vital medications worldwide. To learn more about Regulatory strategy and MAH-based Regulatory imperatives, contact us.