In the healthcare arena, the accurate translation of Regulatory documents is not just a necessity-it's a critical component of successful international drug and medical device approvals. As life sciences companies expand their reach across borders, the challenge of translating complex Regulatory documents while maintaining their scientific integrity and legal compliance becomes increasingly paramount.
The translation of Regulatory documents is associated with potential pitfalls. A single mistranslation can lead to severe consequences, from delayed approvals to patient safety risks. Tracing back to 2007, an incorrectly translated label led to 47 patients receiving improperly implanted knee prostheses, resulting in pain, suffering, and the need for corrective surgeries. Such incidents highlight the critical nature of accurate Regulatory translations and the need for robust best practices.
Best Practices in Regulatory Document Translation:
- Employ Qualified Translators: Select translators with not only linguistic proficiency but also subject matter expertise in life sciences and Regulatory affairs. Ideal candidates should possess degrees such as MDs or PhDs in relevant fields, ensuring familiarity with specific medical terminology.
- Understand the Target Audience: Tailor the translation style to the intended audience, whether healthcare professionals or consumers. This affects the choice of terminology, syntax, and overall presentation.
- Maintain Consistency: Develop and adhere to a standardized terminology database to ensure consistency across all documents, especially crucial for frequently updated materials.
- Leverage Technology Wisely: Utilize AI and machine learning tools to assist with initial drafts and maintain terminology consistency, but always have human experts review and refine the translations.
- Ensure Cultural Competence: Beyond language skills, translators must understand cultural nuances, health literacy levels, and regional medical practices to produce culturally appropriate translations.
- Implement Rigorous Quality Control: Establish a multi-step review process involving proofreading, editing, and in-country review to catch and correct any errors or inconsistencies.
- Preserve Formatting and Structure:Maintain the original document's format, including headings, sections, and numbering, to ensure the translated version mirrors the source document's appearance.
Common Pitfalls to Avoid:
- Literal Translations
- Neglecting Regulatory Requirements
- Overlooking Cultural Sensitivities
- Inconsistent Terminology
- Inadequate Subject Matter Expertise
Table: Comparison of Best Practices and Common Pitfalls
| Best Practices | Common Pitfalls |
|---|---|
| Employ qualified, specialized translators | Relying on general translators without subject expertise |
| Tailor content to the target audience | One-size-fits-all approach to translation |
| Maintain consistent terminology | Inconsistent use of terms across documents |
| Leverage AI tools with human oversight | Over-reliance on machine translation without expert review |
| Ensure cultural competence | Neglecting cultural context and sensitivities |
| Implement rigorous quality control | Inadequate review and proofreading processes |
| Preserve original formatting and structure | Altering document structure during translation |
Benefits of Partnering with Regulatory Translation Experts
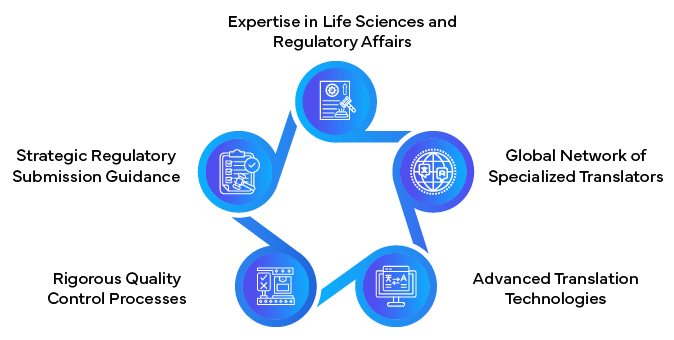
Synopsis:
Translating Regulatory documents is a complex and critical process that demands precision, expertise, and cultural sensitivity. By adhering to best practices and avoiding common pitfalls, life sciences companies can navigate the challenges of global Regulatory submissions more effectively.
Partnering with specialized Regulatory translation services can provide the expertise and resources necessary to ensure accurate, compliant, and culturally appropriate translations. In an industry where precision can mean the difference between approval and rejection—or even impact patient safety—investing in high-quality Regulatory translation is not just advisable; it's essential for global success in the life sciences sector. To learn more such of our Regulatory services click here.