Lifecycle Management (LCM) has become a critical strategy for maintaining product relevance and maximizing value throughout a drug's lifespan in the ever-evolving pharmaceutical landscape. As market dynamics shift and Regulatory landscapes change, innovative approaches to Lifecycle Management are essential for pharmaceutical companies to stay competitive. In this blog, we delve and share more insights on cutting-edge Lifecycle Management strategies and their potential to transform the industry.
Innovative Lifecycle Management Strategies
Leveraging Digital Technologies
The integration of digital technologies is revolutionizing LCM. From artificial intelligence-driven data analysis to blockchain for supply chain management, these tools are enhancing decision-making and operational efficiency.
Key Benefits:
- Real-time data analysis for market trends
- Enhanced predictive modeling for product performance
- Improved supply chain visibility and management
Embracing Personalized Medicine
The shift towards personalized medicine is reshaping Lifecycle Management strategies. By tailoring treatments to individual patient profiles, companies can extend product lifecycles and open new market segments.
Approaches:
- Biomarker-driven drug development
- Companion diagnostics
- Targeted therapies based on genetic profiles
Proactive Regulatory Planning
Innovative Lifecycle Management involves anticipating Regulatory changes and adapting strategies accordingly.
Strategies:
- Early engagement with Regulatory bodies
- Continuous monitoring of global Regulatory trends
- Integration of Regulatory insights into product development
Traditional vs. Innovative LCM Approaches
| Traditional LCM Approaches | Innovative LCM Approaches |
|---|---|
| Reactive Regulatory planning | Proactive Regulatory planning |
| Standardized treatment | Personalized medicine |
| Manual data management | Digital technologies and automation |
(not limited to)
The Role of Regulatory Partners in Innovative Lifecycle Management
Regulatory partners are pivotal in implementing these innovative strategies. They bring expertise in:
- Digital Transformation: Guiding the integration of new technologies into LCM processes.
- Regulatory Intelligence: Providing up-to-date insights on global Regulatory trends.
- Strategic Planning: Helping companies align LCM strategies with long-term business goals.
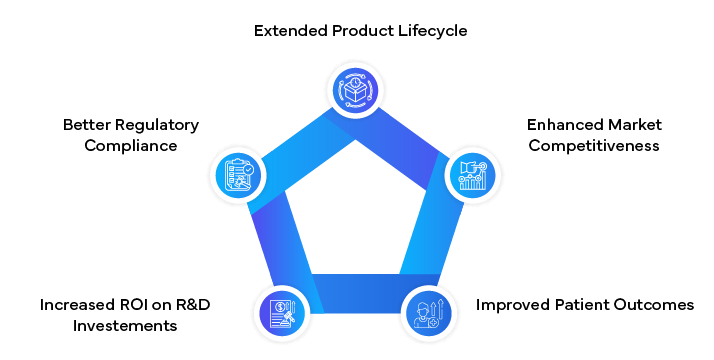
Benefits of Innovative LCM
Conclusion
Innovative approaches to Lifecycle Management are no longer optional but essential for pharmaceutical companies aiming to thrive in a competitive market. By embracing digital technologies, personalized medicine, and proactive Regulatory strategies, companies can ensure their products remain relevant and valuable throughout their lifecycle. As the industry continues to evolve, partnerships with specialized Regulatory experts will become increasingly crucial. These collaborations will enable companies to navigate complex Regulatory landscapes, leverage cutting-edge technologies, and ultimately deliver better outcomes for patients and stakeholders alike.