In the ever-evolving landscape of pharmaceutical development, the journey from laboratory discovery to patient treatment is paved with complex Regulatory challenges. In this process lies the New Drug Application (NDA), a critical milestone encompassing scientific endeavor and investment. This blog delves into the intricacies of NDA Regulatory operations, offering valuable insights and lessons learned from industry veterans.
The NDA Landscape
New Drug Applications represent the culmination of extensive research, development, and clinical testing. They serve as the comprehensive dossier that convinces Regulatory authorities, such as the U.S. Food and Drug Administration (FDA), that a new drug product is safe, effective, and ready for market approval.
The Complexity of the NDA Process
One of the primary hurdles in NDA submissions is the sheer volume and complexity of data required. Pharmaceutical companies must navigate a labyrinth of Regulatory guidelines while presenting clear, compelling evidence of their drug product merits. This balancing act often leads to delays, costly revisions, and in some cases, outright rejections.
To shed light on this critical process, let's break down the key stages of NDA submission and explore strategies for success at each step.
Table 1: Key Stages in NDA Submission
| Stage | Description | Best Practices |
|---|---|---|
| Preclinical Research | Laboratory and animal studies to gather initial data | Ensure robust study design and meticulous data collection |
| Clinical Trials | Human trials conducted in phases (I, II, III) | Implement rigorous protocols and maintain data integrity |
| Documentation | Compilation of safety, efficacy, and quality data | Organize data systematically and ensure clarity in presentation |
| Regulatory Review | Submission to FDA and review process | Maintain open communication with regulatory bodies |
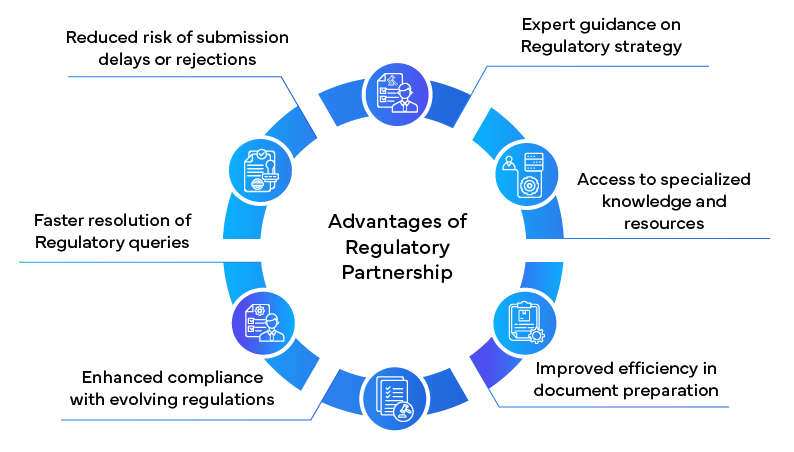
Role of Regulatory Partnership
In navigating the complex NDA landscape, many pharmaceutical companies are turning to Regulatory partners for support. These specialized firms offer a wealth of experience and expertise that can significantly streamline the NDA process.
Lessons Learned
As we look to the future, the importance of effective NDA Regulatory operations will only grow. With the pace of innovation accelerating and Regulatory landscapes evolving, those who master this critical process will be best positioned to bring life-changing treatments to patients around the world.
The path to a successful NDA submission is rarely straightforward, moreover by embracing a strategic approach to Regulatory operations, leveraging expert partnerships, and maintaining a commitment to quality and compliance, pharmaceutical companies can significantly improve their chances of NDA success.