Effective communication with health authorities (HA) is crucial for managing the lifecycle management of pharmaceutical medicinal products. The drug product approval process is complex and involves various stages, each requiring clear and strategic interaction with HA. This blog explores strategies for successful interactions with HA, ensuring smooth and efficient Regulatory processes that can expedite approvals and maintain compliance.
Challenges faced
Navigating HA interactions can be challenging due to varying requirements, expectations, and communication patterns. Unable to comprehend HA queries or delays in responses can lead to significant setbacks in the approval process. Regulatory submissions require precise documentation, timely updates, and strategic negotiations to align with HA guidelines.
The lack of a well-structured interaction strategy can result in prolonged review times, increased costs, and potential non-compliance.
Strategies for Health Authority Interactions
- Early HA engagement
Engaging early with HA during the development process is a proactive strategy that helps in understanding their expectations and requirements. Early engagement can involve HA consultations through correspondence or pre-submission meetings.
Key Actions:
By scheduling HA meetings sponsors can present preliminary data and development plans to receive initial feedback.
- Clear and Concise Communication
Preparing clear and concise documents that address the HA questions and concerns is essential. This includes briefing documents for meetings, detailed study protocols, and responses to queries.
Key Actions:
Develop structured and well-organized briefing documents with the usage of clear-cut language and avoid unnecessary jargon.
Ensure that all questions from health authorities are answered thoroughly and clearly.
- Regulatory Intelligence
Aligning with the latest Regulatory updates, guidelines, and precedents helps in preparing up-to-date submission documents.
Key Actions:
Subscribe to Regulatory newsletters and updates from HA.
Participate in industry conferences and workshops alongside analyzing previous HA feedback and decisions to identify patterns and expectations.
- Pre-Submission Meetings
Conducting pre-submission HA meetings is a strategic move to discuss the submission strategy, identify potential issues, and seek advice.
Key Actions:
Requesting the HAs for pre-submission meetings with a detailed agenda and thereby seeking feedback to refine the submission package.
- Follow-Up and Feedback
Promptly addressing HA feedback and queries is crucial to keeping the review process on track. Effective follow-up involves timely responses to questions, providing additional data or clarifications, and ensuring that all feedback is incorporated into the documents.
Key Actions:
Establish a system for tracking HA feedback and queries from health authorities by prioritizing responses and ensuring that they are provided within the stipulated timelines.
Documenting all the communications and feedback for future reference and compliance.
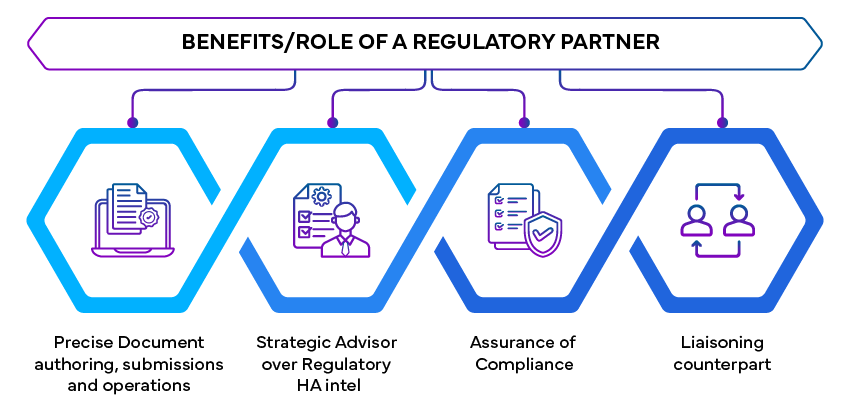
Role of Regulatory Partner
A Regulatory partner can facilitate HA interactions by providing expert guidance and support throughout the submission process. They bring specialized knowledge and experience that can enhance the quality of submissions and streamline interactions.
Summary
Successful HA interactions are vital for Regulatory success. Early engagement, clear communication, and strategic planning are key strategies that can significantly enhance the approval process. Partnering with experienced Regulatory service providers like Freyr can further streamline these interactions, ensuring timely approvals and maintaining compliance throughout the product lifecycle.