Interchangeability with a reference biologic product is a crucial step in the approval process for biosimilars. It ensures that a biosimilar can be substituted for the reference product without any significant risk of reduced efficacy or increased safety concerns. This blog attempts to comprehend the latest FDA update on the considerations for achieving interchangeability, and industry best practices in achieving Regulatory excellence.
Defining Interchangeability
Achieving interchangeability is a complex and rigorous process that requires meeting stringent Regulatory requirements. The challenge lies in demonstrating that the biosimilar not only matches the reference product in terms of safety, and efficacy but also provides the same clinical results in any given patient. Failure to meet these requirements can result in delays or denials in approval, affecting market access and patient care.
Regulatory Requirements for Interchangeability
The FDA outlines specific criteria for demonstrating interchangeability in its guidance documents. These criteria include:
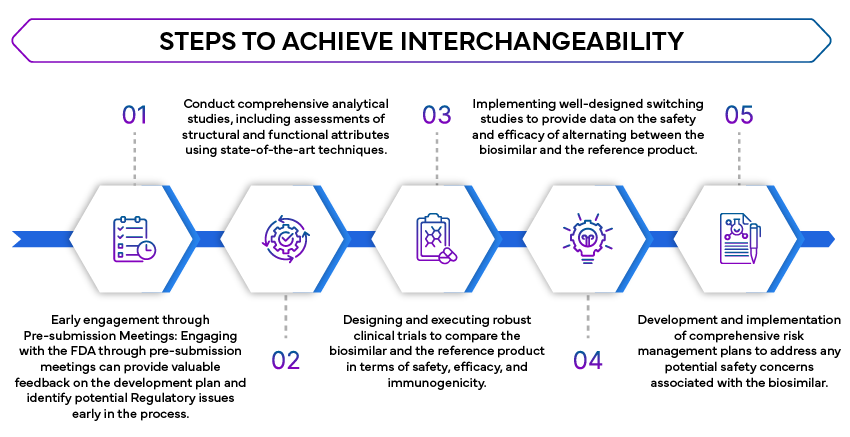
- Comparative Analytical Studies: Extensive analytical studies are required to demonstrate that the biosimilar is highly similar to the reference product. These studies assess the molecular structure, function, and composition of the biosimilar.
- Clinical Studies: Clinical studies are needed to confirm that the biosimilar has no clinically meaningful differences from the reference product in terms of safety, purity, and potency. This often involves comparative pharmacokinetic (PK) and pharmacodynamic (PD) studies.
- Switching Studies: The FDA requires switching studies to evaluate the impact of alternating between the biosimilar and the reference product. These studies are crucial to ensure that the switch does not affect the efficacy or safety of the treatment.
- Risk Evaluation and Mitigation Strategies (REMS): If applicable, REMS must be addressed to ensure that the biosimilar can be used interchangeably without additional safety concerns.
Steps to achieve interchangeability strategically
Role of a Regulatory Partner
Regulatory partnerships can significantly enhance the likelihood of achieving interchangeability. Here is how a Regulatory partner can help:
- Expert Guidance: Provides expert advice on Regulatory requirements and strategies to meet FDA guidelines.
- Documentation Support: Assists in the preparation of high-quality submission documents that comply with Regulatory standards.
- Clinical Study Design: Supports the design and execution of robust clinical and switching studies.
- HA Interactions: Facilitates effective communication with HA, ensuring timely and constructive feedback.
- Risk Management: Develop comprehensive risk management strategies to mitigate potential safety concerns.
Advantages of Having a Regulatory Partner
| Advantages | Description | |
| 1. | Expertise in Regulatory Requirements | Deep understanding of global regulatory landscapes and requirements. |
| 2. | Streamlined Documentation | Ensures accurate and compliant documentation preparation. |
| 3. | Efficient Submission Process | Reduces time and cost through efficient submission management. |
| 4. | Enhanced Compliance | Minimizes risk of delays and rejections through thorough compliance. |
| 5. | Effective Risk Management | Proactive identification and mitigation of potential risks. |
Summary
Achieving interchangeability with a reference product is a demanding but essential process for the successful approval of biosimilars. It requires a comprehensive understanding of Regulatory requirements, meticulous planning, and robust clinical data. Partnering with an experienced Regulatory service provider can greatly enhance the chances of success, ensuring that the biosimilar meets the stringent criteria set by Regulatory authorities. By leveraging expert guidance, thorough documentation, and strategic risk management, sponsors can navigate the complex pathway to interchangeability more effectively.