Post-approval change management is an essential, yet often overlooked aspect of pharmaceutical product lifecycle management. Once a drug product has been approved, it must continue to meet Regulatory standards to ensure its safety, efficacy, and quality. This involves making necessary changes to the product, its manufacturing process, or its packaging.
These changes often range from improving production efficiency, addressing safety issues, or complying with latest and/ or new Regulatory requirements. However, managing these changes presents several hidden challenges that can impact the product's lifecycle and market presence. In such an endeavor, it necessitates the presence of a Regulatory partner to look upon such change process management and drive the Regulatory operations smoothly.
Hurdles for a pharmaceutical company
Navigating post-approval changes follows complexities that significantly across different regions, creating compliance hurdles. Additionally, ensuring data integrity, coordinating multiple stakeholders, and managing extensive documentation can be daunting. Failure to effectively manage these aspects can result in Regulatory non-compliance, product recalls, and severe reputational and operational damage.
Few challenges in achieving Post-Approval Change Management
- Regulatory Complexity
- Different health authorities (HA) around the world have unique requirements for post-approval changes that necessitate a deep understanding of each region’s specific guidelines and timelines. Understanding these differences is crucial for timely and successful submissions.
- Delays in comprehending these regulations can lead to significant setbacks in the product’s lifecycle.
- Data Integrity
- Ensuring the accuracy and integrity of data submitted for post-approval changes is paramount. Inaccurate or incomplete data can lead to Regulatory rejections and significant delays.
- Maintaining high data quality standards involves rigorous validation processes and frequent audits to prevent discrepancies.
- Stakeholder Coordination
- Effective post-approval change management involves coordination among internal teams (such as R&D, manufacturing, and quality assurance) and external partners (such as contract manufacturing organizations).
- Using project management tools and regular cross-functional meetings can enhance collaboration and ensure that all parties are aligned.
- Documentation
- Detailed and precise documentation is crucial for demonstrating compliance and facilitating smooth review processes.
- On the contrary, poor documentation practices can lead to Regulatory queries, additional data requests, and submission delays.
- Risk Management
- Proactive risk management helps in anticipating challenges and implementing solutions to keep the submission process on track.
- An effective risk management plan minimizes the likelihood of Regulatory setbacks and ensures ongoing compliance.
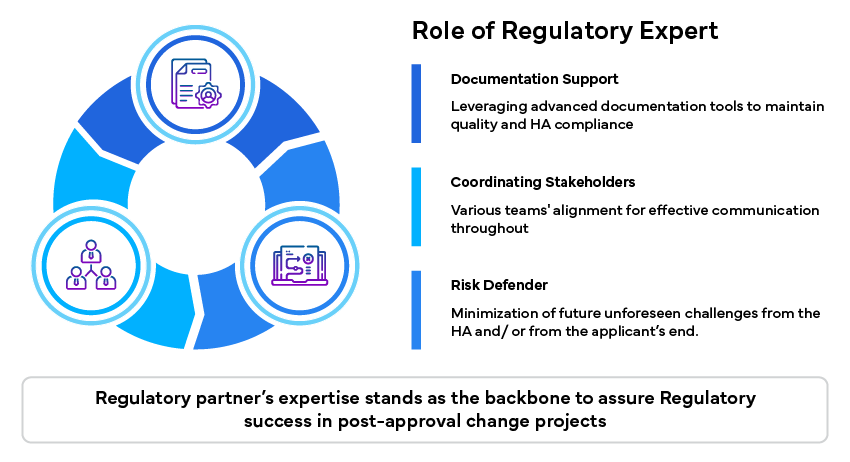
Role of Regulatory Expert
Regulatory experts play a significant role in overcoming these challenges by providing specialized expertise and support:
Summary
Effective post-approval change management is essential for maintaining the quality, safety, and efficacy of pharmaceutical drug products. Navigating Regulatory complexities is necessary to achieve change management approvals from the HA. Regulatory experts provide invaluable support in overcoming these challenges by offering their expertise, facilitating coordination, and mitigating risks. By leveraging their capabilities applicants can ensure successful post-approval change management and maintain their products' market presence.