Handling and managing the dynamic Regulatory landscape are a critical task for life sciences companies who aim to bring their products to market. A well-structured Regulatory submission roadmap can streamline a company’s Regulatory processes that will ensure compliance and expedite health authority (HA) approvals ahead. This blog delves into the challenges necessitated for the creation of Regulatory submission roadmaps, highlighting key steps of these roadmaps, and the role of having a Regulatory partner in critical operations.
Developing a Regulatory submission roadmap involves challenges which could be;
- Understanding Regulatory requirements across different regions,
- Managing timelines, and
- Coordinating various stakeholders.
Without a clear roadmap, companies risk delays, non-compliance, and increased operational costs. The intricacies of Regulatory requirements, frequent updates to guidelines, and the necessity for cross-functional collaboration often led to delays.
Creating Regulatory Submission Roadmaps:
Creating a Regulatory submission roadmap involves several key steps:
- Regulatory landscape analysis
It’s essential to understand the specific requirements of Regulatory authorities in target markets such as the FDA, EMA, and Health Canada. This involves reviewing guidelines, regulations, and models to ensure the roadmap aligns with the latest Regulatory expectations. This analysis forms the foundation for identifying critical submission milestones and compliance checkpoints.
Consulting Regulatory experts who have a deep understanding of the Regulatory landscape can provide valuable insights. These experts can help interpret complex regulations and identify potential hurdles early in the process.
- Gap Analysis
Identifying data and documentation gaps helps in planning necessary studies or generating additional data. A thorough gap analysis ensures that all Regulatory requirements are met, reducing the risk of submission rejection or delays.
- Timeline Development
Establishing a detailed timeline that includes all critical milestones, from pre-submission meetings to final submission ensures that all stakeholders are aware of their roles and deadlines, facilitating smooth coordination and timely submissions.
Regular monitoring of progress against the timeline helps ensure that the project stays on track. This involves setting up regular check-ins and updates to track milestones and address any delays promptly.
- Stakeholder Coordination
Collaborating with internal teams (R&D, clinical, quality assurance) and external partners (CROs, CMOs) to gather necessary data and documentation. Effective coordination ensures that all required information is collected, reviewed, and incorporated into the submission package promptly.
- Risk Management
Identifying potential risks (e.g., delays in data generation, changes in Regulatory guidelines) and developing mitigation strategies. Proactive risk management helps in anticipating challenges and implementing solutions to keep the submission process on track.
Developing contingency plans for potential setbacks ensures that the project can proceed smoothly even if unexpected issues arise. This includes having backup plans for critical activities and resources.
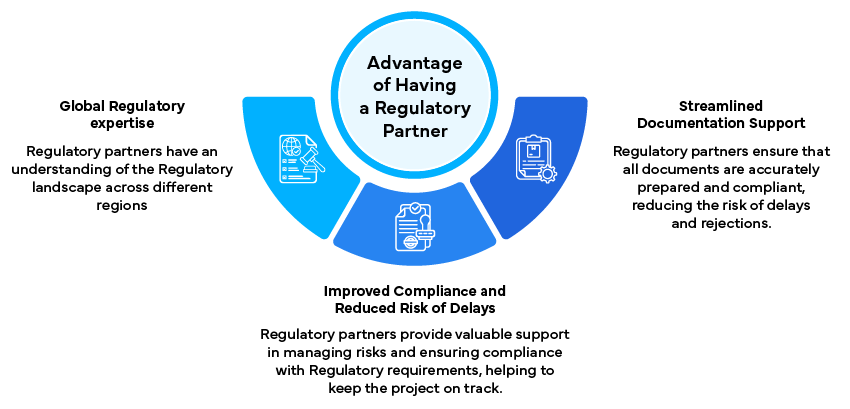
Role of Regulatory Partner
A Regulatory partner plays a vital role in the creation and execution of submission roadmaps:
- Expert Guidance: Provides insights into the Regulatory landscape and helps interpret guidelines, ensuring that the submission strategy is aligned with Regulatory expectations. Regulatory partners have the experience and expertise to navigate complex regulations and anticipate potential issues.
- Document Management: Ensures all necessary documents are accurately prepared and compliant, leveraging expertise in Regulatory writing and document control. This includes reviewing documents for accuracy, completeness, and compliance with Regulatory requirements.
- Timeline Adherence: Monitors progress and ensures adherence to the established timeline, using project management tools and techniques to track milestones and deadlines. Regular progress updates and proactive problem-solving help keep the project on track.
- Risk Mitigation: Identifies potential risks early and develops strategies to address them, ensuring that the submission process remains on schedule. Regulatory partners can provide valuable support in developing and implementing risk mitigation plans.
Summary
A well-crafted Regulatory submission roadmap is essential for successful product approval. It helps in navigating the complex Regulatory landscape, ensuring compliance, and minimizing delays. Partnering with an experienced Regulatory service provider can significantly enhance the efficiency and effectiveness of this process, providing valuable support in managing risks, coordinating stakeholders, and ensuring compliance with Regulatory requirements.